1. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016; Dec. 3. 388(10061):2783–95.

2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
3. Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: a review and comparison. Ann Surg. 2007; 245:366–78.
4. Edge SB. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed. New York: Springer;2016.
5. Pitoia F, Jerkovich F, Urciuoli C, Schmidt A, Abelleira E, Bueno F, et al. Implementing the modified 2009 American Thyroid Association risk stratification system in thyroid cancer patients with low and intermediate risk of recurrence. Thyroid. 2015; 25:1235–42.

6. Palmer CR. Encyclopedia of biostatistics. BMJ. 1999; 318:542.

7. Ikeda M, Ishigaki T, Yamauchi K. Relationship between Brier score and area under the binormal ROC curve. Comput Methods Programs Biomed. 2002; 67:187–94.

8. Guo K, Wang Z. Risk factors influencing the recurrence of papillary thyroid carcinoma: a systematic review and meta-analysis. Int J Clin Exp Pathol. 2014; 7:5393–403.
9. Chen Y, Li Y, Zhou X. BRAF mutation in papillary thyroid cancer: a meta-analysis. Int J Clin Exp Med. 2016; 9:13259–67.
10. Yin DT, Yu K, Lu RQ, Li X, Xu J, Lei M, et al. Clinicopathological significance of TERT promoter mutation in papillary thyroid carcinomas: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2016; 85:299–305.

11. Malandrino P, Russo M, Regalbuto C, Pellegriti G, Moleti M, Caff A, et al. Outcome of the diffuse sclerosing variant of papillary thyroid cancer: a meta-analysis. Thyroid. 2016; 26:1285–92.

12. Yang J, Gong Y, Yan S, Shi Q, Zhu J, Li Z, et al. Comparison of the clinicopathological behavior of the follicular variant of papillary thyroid carcinoma and classical papillary thyroid carcinoma: a systematic review and meta-analysis. Mol Clin Oncol. 2015; 3:753–64.

13. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97:418–28.

14. Yildirim E. A model for predicting outcomes in patients with differentiated thyroid cancer and model performance in comparison with other classification systems. J Am Coll Surg. 2005; 200:378–92.

15. Tavares C, Melo M, Cameselle-Teijeiro JM, Soares P, Sobrinho-Simoes M. Endocrine tumours: genetic predictors of thyroid cancer outcome. Eur J Endocrinol. 2016; 174:R117–26.

16. Pak K, Lee SH, Lee JG, Seok JW, Kim IJ. Comparison of visceral fat measures with cardiometabolic risk factors in healthy adults. PLoS One. 2016; 11:e0153031.

17. Lee SG, Lee WK, Lee HS, Moon J, Lee CR, Kang SW, et al. Practical performance of the 2015 American Thyroid Association guidelines for predicting tumor recurrence in patients with papillary thyroid cancer in South Korea. Thyroid. 2017; 27:174–81.

18. Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, et al. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid. 2016; 26:373–80.

19. Brierley JD, Panzarella T, Tsang RW, Gospodarowicz MK, O’Sullivan B. A comparison of different staging systems predictability of patient outcome. Thyroid carcinoma as an example. Cancer. 1997; 79:2414–23.
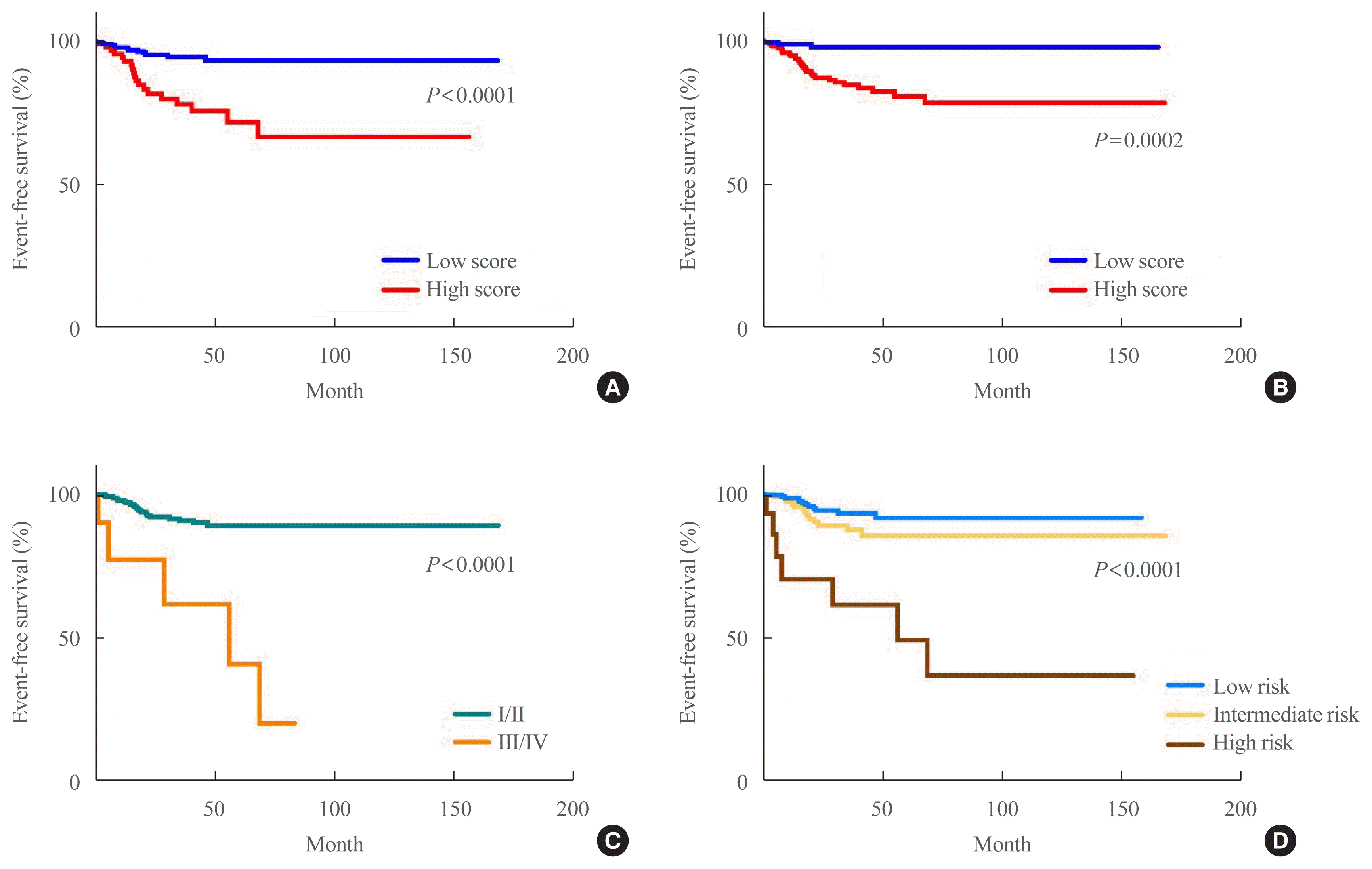
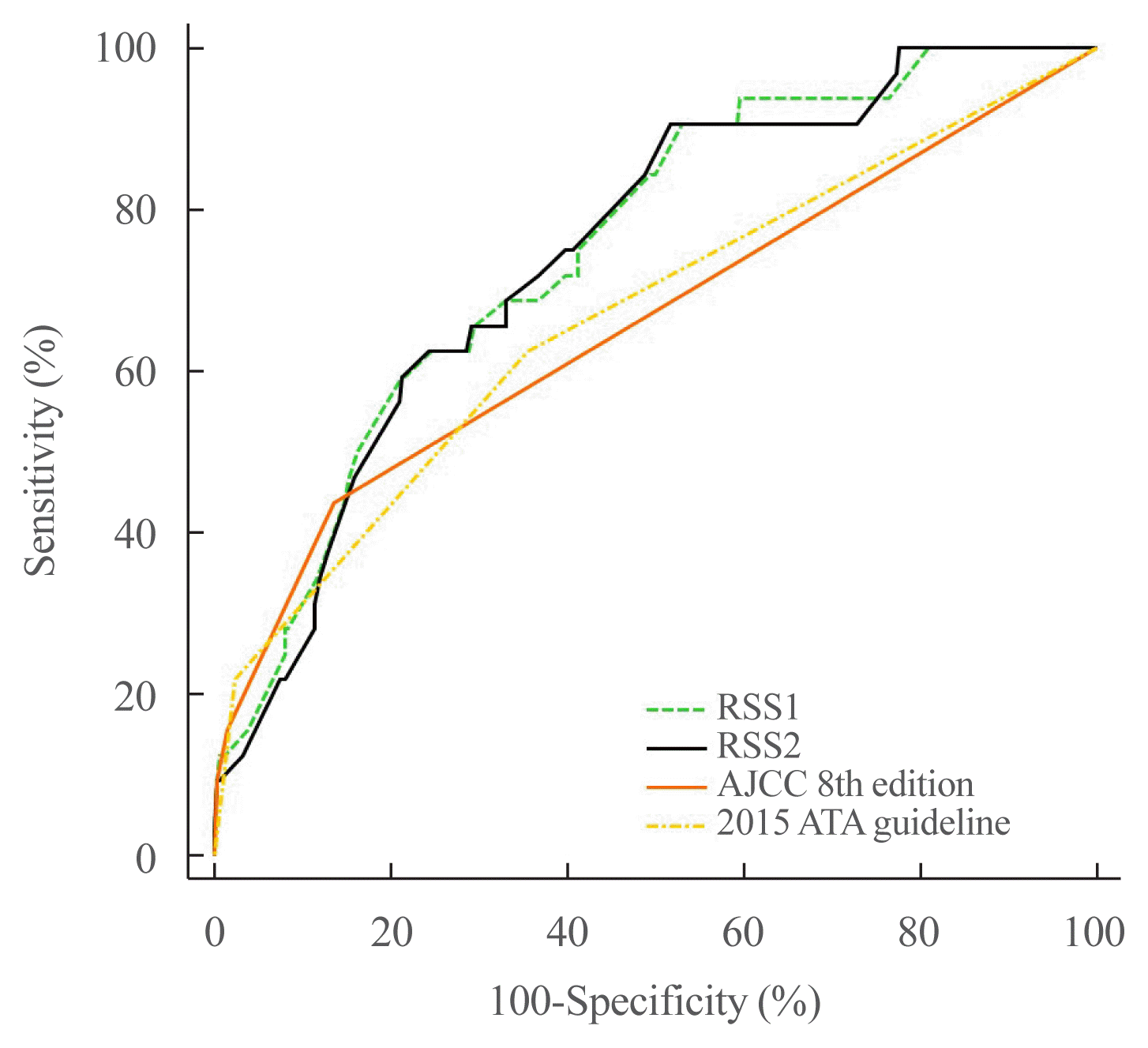
20. Pak K, Kim YH, Suh S, Goh TS, Jeong DC, Kim SJ, et al. Development of a risk scoring system for patients with papillary thyroid cancer. J Cell Mol Med. 2019; 23:3010–5.

21. Boos LA, Schmitt A, Moch H, Komminoth P, Simillion C, Marinoni I, et al. MiRNAs are involved in tall cell morphology in papillary thyroid carcinoma. Cancers (Basel). 2019; 11:885.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download