INTRODUCTION
METHODS
Literature search
Inclusion criteria
Exclusion criteria
Data extraction
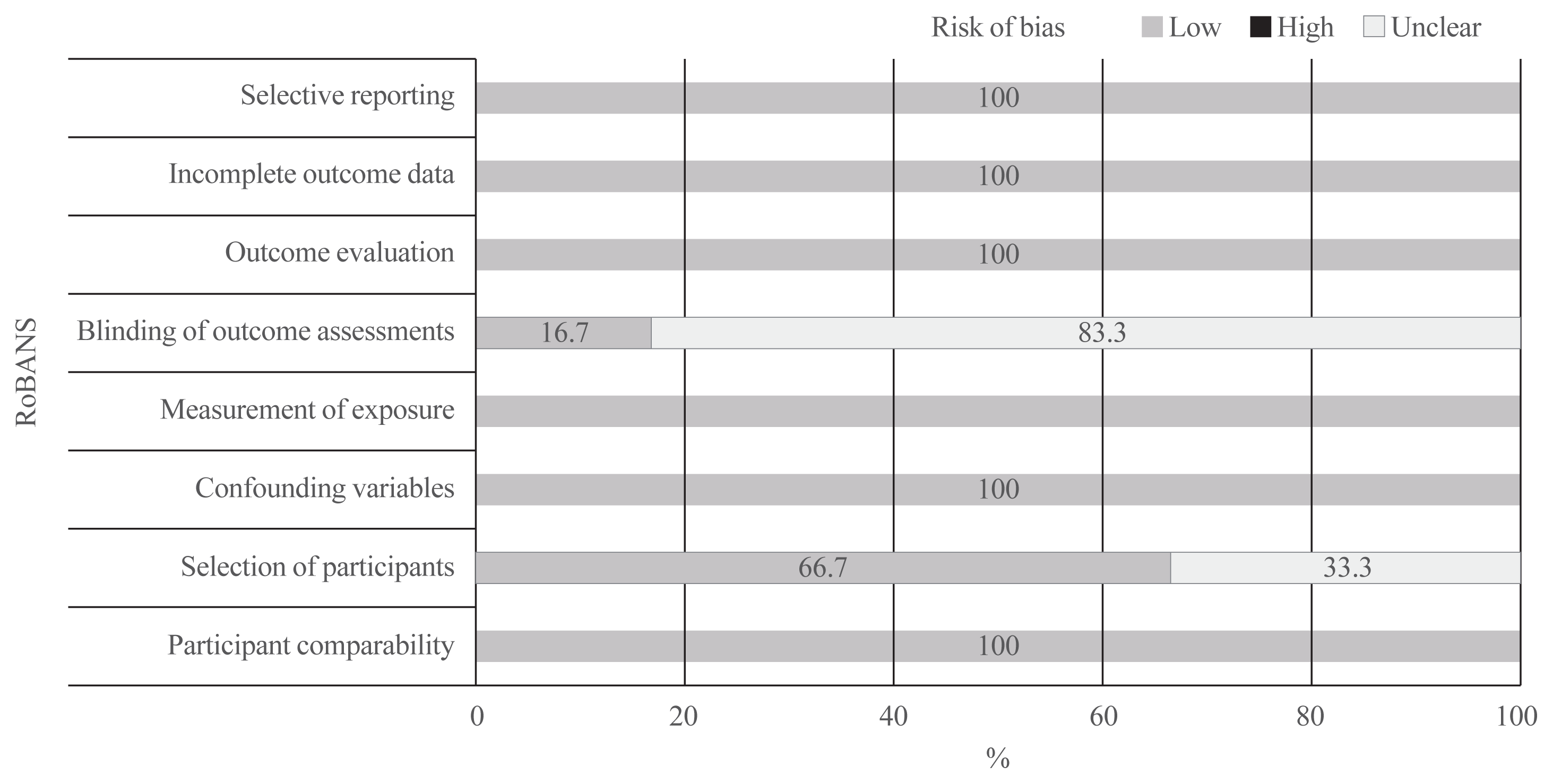
Quality assessment
Data synthesis and analyses
RESULTS
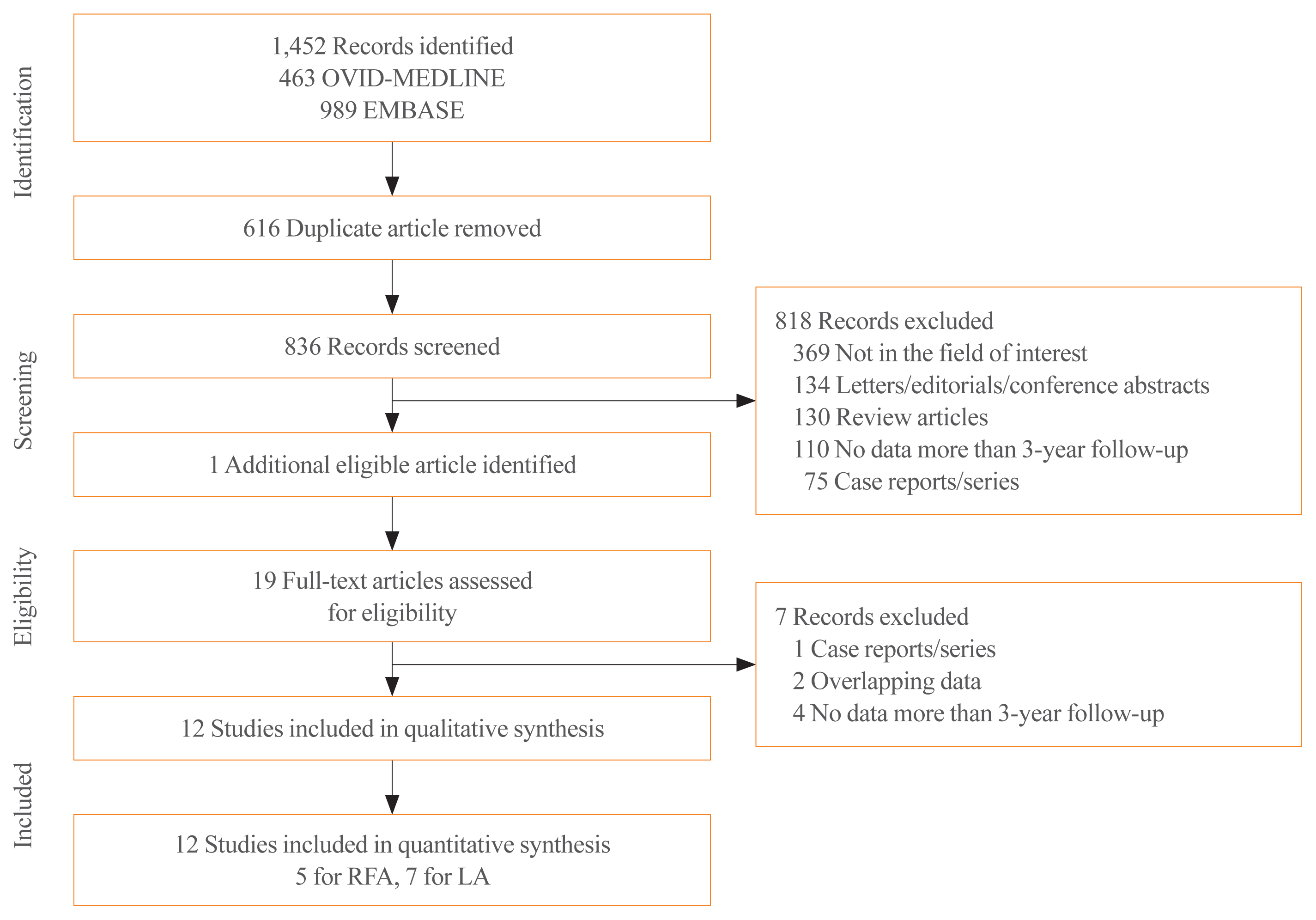
Literature search
Characteristics of the included studies
Table 1
| Group | Study | Enrollment period | Affiliation | Patient no. | Mean age | Male: female | Study design | Nodule no. | Criteria for ablation (summary of inclusion and exclusion criteria) |
|---|---|---|---|---|---|---|---|---|---|
| RFA | Aldea Martinez et al. (2019) [10] | May 2014 to Oct 2018 | Hospital Universitario de Burgos, Spain | 24 | 50.2 | 4:20 | Retro. | 24 |
Inclusion: symptomatic nodule, TIRADS 1 or 2, 2 consecutive Bethesda II, solid or predominantly solid nodules Exclusion: contralateral laryngeal nerve palsy, presence of pacemaker, pregnancy, cystic nodules, history of neck radiation, patient’s refusal |
| RFA | Deandrea et al. (2019) [11] | 2011 to 2015 | Mauriziano Hospital, Italy | 215 | 66 | 33:182 | Retro. | 215 |
Inclusion: solid or mixed with a solid portion >70%, benign (2 consecutive Bethesda II), normal thyroid function Exclusion: RFA performed more than once |
| RFA | Jung et al. (2018) [12] | May 2010 to Dec 2011 | Multicenter, Koreaa | 276 | 46 | 43:302 | Pro. | 276 |
Inclusion: symptomatic or cosmetic problems, 2 consecutive benign cytology results, no malignant feature on US, solid (>50% solid components), predominantly cystic (10%<solid components <50%), normal thyroid function Exclusion: follicular neoplasm, primary thyroid cancer, pregnancy, history of neck radiation, cystic nodules (<10% solid components) |
| RFA | Lim et al. (2013) [13] | Jun 2002 to Dec 2007 | Daerim St. Mary’s Hospital, Korea | 111 | 37.9 | 10:101 | Retro. | 126 |
Inclusion: symptomatic or cosmetic problems, size >2 cm, 2 consecutive benign cytology results, no malignant feature on US, normal thyroid function, refused or ineligible for surgery Exclusion: NA |
| RFA | Sim et al. (2017) [14] | Jun 2008 to Nov 2013 | Withsim Clinic, Korea | 54 | 44.1 | 5:49 | Retro. | 54 |
Inclusion: symptomatic or cosmetic problems, at least 12 months of follow-up Exclusion: NA |
| LA | Dossing et al. (2011) [3] | 1999 to 2008 | Odense University Hospital, Denmark | 78 | 46 | 4:74 | Retro. | 78 |
Inclusion: symptomatic or cosmetic problems, solitary, solid, cold nodule, benign cytology, normal thyroid function, normal calcitonin Exclusion: family history of thyroid cancer, history of neck radiation |
| LA | Gambelunghe et al. (2013) [4] | 2005 to 2008 | University of Perugia, Italy | 40 | 63 | NA | Retro. | 40 |
Inclusion: symptomatic nodule, single or dominant nodule, benign cytology, cold nodule, normal thyroid function, normal calcitonin, solid, refused or for ineligible surgery, size of 5–150 mL Exclusion: NA |
| LA | Gambelunghe et al. (2018) [5] | 2009 to 2014 | University of Perugia, Italy | 82 | 41 | 31:51 | Retro. | 82 |
Inclusion: toxic nodule, hot nodule, normal thyroglobulin, normal calcitonin, normal anti-thyroglobulin antibodies and anti-microsomal antibodies, benign cytology, refused or ineligible for surgery or other conventional treatment Exclusion: history of neck radiation and surgery |
| LA | Magri et al. (2020) [6] | Jan 2010 to Jan 2013 | Istituti Clinici Scientifci Maugeri IRCCS, Italy | 43 | 55.9 | 5:38 | Retro. | 43 |
Inclusion: symptomatic or cosmetic problems, single or dominant nodule, benign cytology (TIR 2) Exclusion: NA |
| LA | Negro et al. (2019) [7] | Jul 2009 to Mar 2012 | V. Fazzi Hospital, Italy | 62 | 54.7 | 15:47 | Retro. | 62 |
Inclusion: symptomatic or cosmetic problems, 2 consecutive benign cytology results, cold nodule, normal thyroid function, normal calcitonin Exclusion: history of thyroid treatment, thyroid hormone-related drug treatment, history of neck radiation |
| LA | Papini et al. (2014) [8] | Unclear, over 1 year | Multicenter, Italyb | 101 | 51.5 | 16:85 | Pro. | 101 |
Inclusion: solid, 2 consecutive benign cytology results, normal thyroid function, cold nodule, maximum diameter >30 mm, volume of 6–17 mL Exclusion: autoimmunity, previous thyroid gland treatment, thyroid hormone-related drug treatment, history of neck radiation |
| LA | Valcavi et al. (2010) [9] | Jan 2004 to Dec 2006 | Arcispedale Santa Maria Nuova, Italy | 122 | 52.2 | 27:95 | Retro. | 122 |
Inclusion: symptomatic or cosmetic problems, 2 consecutive benign cytology results, single or dominant nodule, solid or mixed (<20% cystic components), size of 2.5–90 mL, diameter >1.5 cm and <7 cm, cold nodule, normal thyroid function, normal calcitonin, no malignant feature on US, normal platelet count and blood coagulation tests Exclusion: family history of thyroid cancer, history of neck radiation and surgery |
Characteristics of the ablation methods
Table 2
| Group | Study | Ablation device | Mean power, W; Mean energy, J | Applicator | Mean ablation time, min | Sessions | Mean ablation session (once: multiple) | Technique | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Local anesthesia or sedation | Trans-isthmic approach | Moving-shot technique | Pull-back technique | ||||||||
| RFA | Aldea Martinez et al. (2019) [10] | AMICA-GEN AGN-H-1.0 programmable RF generator (LOGSA Endomedical Group, Italy) | 45.8 W; NA | 18 G internal cooled amicaeditor probe, 1 cm or 1.5 cm active tip | 15.6 | Multiple | 3.5 (NA:yes) | Lidocaine | Yes | Yes | NA |
| RFA | Deandrea et al. (2019) [11] | RFA needle tube system (RF Medical Seoul Korea or VIVA STARmed, Korea) | 55 W; NA | 18 G internal cooled electrode, 1 cm active tip | 14 | Single | 1 (215:0) | 2% lidocaine | Yes | Yes | NA |
| RFA | Jung et al. (2018) [12] | RF generators, Cool-Tip RF system (Covidien, Boulder, CO, USA; SSP-2000, Taewoong Medical, Korea; and M-1004, RF Medical, Korea) | 78.8 W; 4,161.5 J | 18 G internally cooled electrode, 0.7, 1, 1.5, and 2 cm active tips | 9.5 | Multiple | 1.3 (206:70) | 1%–2% lidocaine | Yes | Yes | NA |
| RFA | Lim et al. (2013) [13] | RF generator, Cool-Tip RF system (Covidien; SSP-2000, Taewoong Medical, Korea) | NA; 21,085 J | 17 or 18 G internally cooled electrode, 1, 1.5, and 2 cm active tips | NA | Multiple | 2.2 (53:73) | 2% lidocaine | Yes | Yes | NA |
| RFA | Sim et al. (2017) [14] | RF generators (RF150 and RF 300, Apro-Korea, Korea) | NA; NA | Straight-type modified internally cooled electrodes, 0.5, 0.7, 1, and 1.5 cm active tips | NA | NA | NA (NA:yes) | 2% lidocaine | Yes | Yes | NA |
| LA | Dossing et al. (2011) [3] | 820 nm continuous-wave infrared diode (Diomed, England) | 1.5–3.5 W; 2,100 J (242 J/mL) | 400 μm laser fiber | 15 | Multiple | 1 (57:21) | 10 mg/mL lidocaine | NA | NA | Yes |
| LA | Gambelunghe et al. (2013) [4] | 1,064 nm continuous-wave Nd-YAG laser (Smart 1064, Elesta, Italy) | 3 W; (245 J/mL) | 300 μm quartz laser fiber | 13.5 | Single | 1 (40:0) | 2.5 mg midazolam (IV) | NA | NA | Yes |
| LA | Gambelunghe et al. (2018) [5] | 1,064 nm continuous-wave Nd-YAG laser (Smart 1064, Elesta, Italy) | 3 W; 1,400–1,800 J | 300 μm quartz laser fiber | NA | Single | 1.2 (82:0) | No | NA | NA | Yes |
| LA | Magri et al. (2020) [6] | 1,064 nm continuous-wave diode laser (Quanta D-Plus, Italy) with an optical beam-splitting device | 2–3 W; 5,084.8 J (336.5 J/mL) | 400 μm quartz laser fiber | 5–10 | Multiple | >1 (34:9) | 1% lidocaine+ 2.5 mg midazolam | NA | NA | Yes |
| LA | Negro et al. (2019) [7] | 1,064 nm Nd-YAG laser (Echolaser, Italy) | 3 W; 6,168 J | 300 μm quartz laser fiber | NA | Multiple | 1 (55:7) | 2% xylocaine+ 2.5 mg midazolam | NA | NA | Yes |
| LA | Papini et al. (2014) [8] | 1,064 nm Nd-YAG laser (Echolaser, Italy) | 3 W; 3,600 or 7,200 J | 300 μm quartz laser fiber | NAa | Single | 1 (101:0) | 2% xylocaine | NA | NA | Yes |
| LA | Valcavi et al. (2010) [9] | 1,064 nm continuous-wave diode laser with an optical beam-splitting device (DEKA, Italy) | 3.1 W; 8,522 J | 400 μm laser fiber | 19.4 | Single | 1 (122:0) | 2% lidocaine+ 2–10 mg diazepam or 2–5 mg midazolam (IV) | NA | NA | Yes |
Results of ablation
Fig. 2
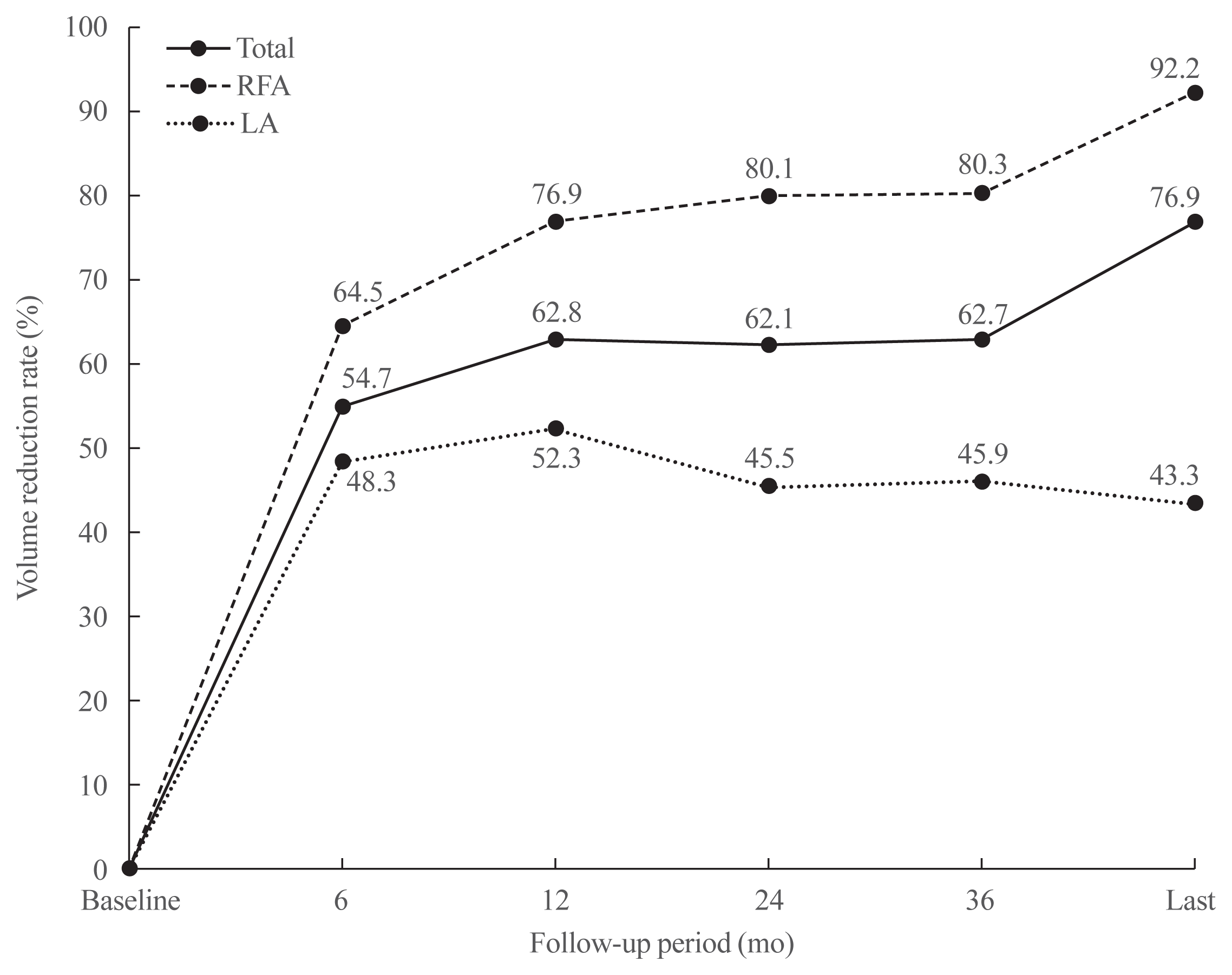
Table 3
| Group | Study | Index nodule | VRR, % | Complications (minor and major)a | No. of patients with delayed surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Volume, mL (range) | Cosmetic score | Symptomatic score | 6 mo | 12 mo | 24 mo | 36 mo | Last (mean mo) | ||||
| RFA | Aldea Martinez et al. (2019) [10] | 36.3 (0.7–231.7) | NA | NA | 56.82 | 68.76 | 69.92 | 76.84 | NA | 4 (3, 1) of 24 | NA |
|
|
|||||||||||
| RFA | Deandrea et al. (2019) [11] | 20.9 (15–33) | 3 of 4-point scaleb | 5 of 10-point scalec | 56.2 | 63 | 67.4 | 66.7 | 66.9 (60) | 6 (6, 0) of 215 | NA |
|
|
|||||||||||
| RFA | Jung et al. (2018) [12] | 14.2 (1.1–80.8) | 3.7 of 4-point scaleb | 2.5 of 10-point scalec | 69.1 | 80.3 | 84.3 | 89.2 | 95.3 (60) | 12 (11, 1) of 276 | 0 |
|
|
|||||||||||
| RFA | Lim et al. (2013) [13] | 9.8 (2–43) | 3.2 of 4-point scaleb | 4.3 of 10-point scalec | 70.3 | 89.9 | 90.1 | 90.7 | 93.5 (NA) | 3 (1, 2) of 111 | NA |
|
|
|||||||||||
| RFA | Sim et al. (2017) [14] | 14 (3.1–56.6) | NA | NA | NA | 73.6 | NA | 69.6 | 97.9 (84) | 2 (1, 1) of 56 | NA |
|
|
|||||||||||
| Pooled proportions for RFA (95% CI) | 16.2 | 64.5 (56.1–72.1) | 76.9 (65–85.7) | 80.1 (66.4–89.2) | 80.3 (66–89.5) | 92.2 (71.9–98.2) | 4.6 (3.6, 1.3) (2.5–8.4) | 0 | |||
|
|
|||||||||||
| LA | Dossing et al. (2011) [3] | 8.2 (2–25.9) | In 46/78 patients | In 74/78 patient | NA | 57.3 | NA | NA | 51 (69) | 0 | 27 of 78 |
|
|
|||||||||||
| LA | Gambelunghe et al. (2013) [4] | 14 (7–142) | NA | NA | 39.3 | 32.1 | 17.9 | 17.9 | NA | 2 (2, 0) of 40 | NA |
|
|
|||||||||||
| LA | Gambelunghe et al. (2018) [5] | 12 (5–118) | In 5/82 patients | In 5/82 patients | 57 | 57 | NA | 58 | NA | 0 | NA |
|
|
|||||||||||
| LA | Magri et al. (2020) [6] | 21.1 | NA | NA | 38 | 41.3 | 36.7 | 33.4 | 25.1 (72) | 0 | 10 of 43 |
|
|
|||||||||||
| LA | Negro et al. (2019) [7] | 15.7 | NA | NA | 52.2 | 56.1 | 57 | 56.5 | 51.1 (60) | NA | 6 of 62 |
|
|
|||||||||||
| LA | Papini et al. (2014) [8] | NA | In 73/101 patient | In 38/101 patient | 49 | 59 | 60 | 57 | NA | 1 (0, 1) of 101 | NA |
|
|
|||||||||||
| LA | Valcavi et al. (2010) [9] | 23.1 | In 46/78 patients | In 74/78 patient | 47.5 | 50.6 | 51.6 | 47.8 | NA | 33 (22, 11) of 122 | NA |
|
|
|||||||||||
| Pooled proportions for LA (95% CI) | 16.1 | 48.3 (43.1–53.6) | 52.3 (46.1–58.5) | 45.5 (33.1–58.5) | 45.9 (35.3–57) | 43.3 (28.9–59) | 2.4 (2, 1.8) (0.5–11.5) | 21.4 (10.1–39.8) | |||
|
|
|||||||||||
| Pooled proportions, total (95% CI) | 16.1 | 54.7 (47.5–61.8) | 62.8 (53.3–71.4) | 62.1 (47.5–74.8) | 62.7 (49–74.6) | 76.9 (54.9–90.1) | 3.8 (2.8, 1.5) (1.6–9.1) | 12.8 (4.2–32.8) | |||
RFA, radiofrequency ablation; M, months; NA, not available; CI, confidence interval; LA, laser ablation.
a Classified according to the Society of Interventional Radiology: major complications were defined as adverse events associated with substantial morbidity or disability, an increased level of care, hospital admission, or substantial prolongation of hospital stay. Minor complications were defined as all other complications; and the others were considered as side effects without presentation in the Table (Major complication: Aldea Martinez et al. (2019) [10], one laryngeal nerve damage; Jung et al. (2018) [12], one hyperthyroidism; Lim et al. (2013) [13], one voice change and one brachial plexus injury; Sim et al. (2017) [14], one voice change during 3 months; Papini et al. (2014) [8], one voice change; Valcavi et al. (2010) [9], six pseudocyst formations, three pseudocysts with fasciitis, and two laryngeal dysfunctions);




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download