INTRODUCTION
METHODS
Subjects
Measurements
Calculation of the HGI
Statistical analyses
RESULTS
Baseline characteristics
Table 1
DM, diabetes mellitus; SBP, systolic blood pressure; DPP4, dipeptidyl peptidase 4; SGLT2, sodium-glucose cotransporter 2; BMI, body mass index; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment for insulin resistance; HOMA-B, homeostatic model assessment of β-cell function.
Table 2
Q, quartile; DM, diabetes mellitus; DPP4, dipeptidyl peptidase 4; SGLT2, sodium-glucose cotransporter 2; BMI, body mass index; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment for insulin resistance; HOMA-B, homeostatic model assessment of β-cell function.
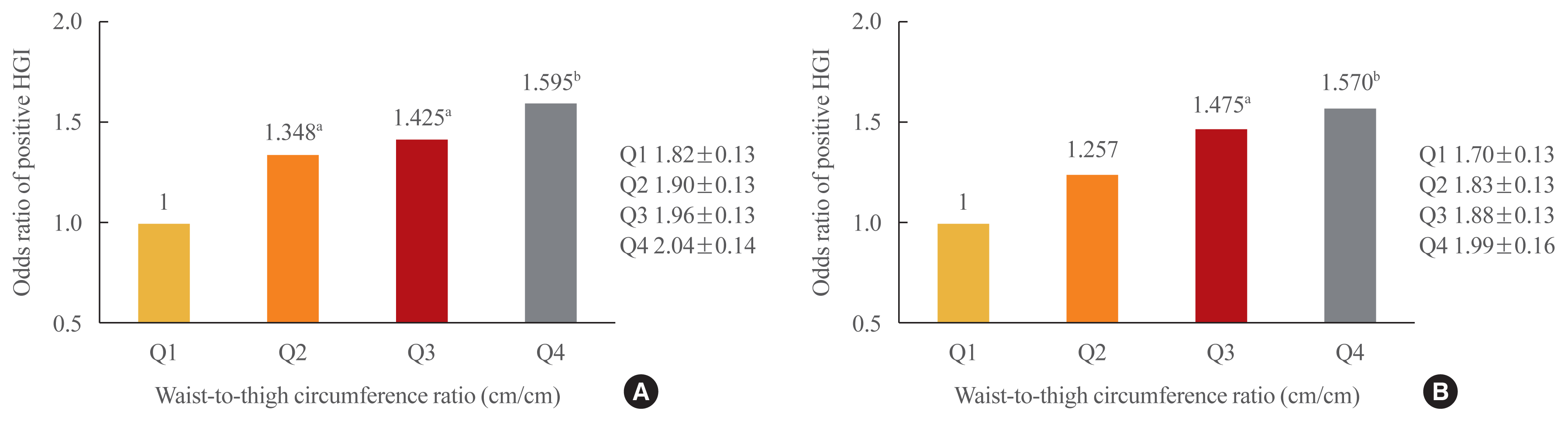
Relative risk of a positive HGI according to thigh and waist circumference
Table 3
| Variable | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| Men | ||||
| Number of subjects | 147 | 709 | 545 | 168 |
| Positive HGI | ||||
| Unadjusted OR | 1.000 | 0.825 (0.577–1.177) | 0.903 (0.626–1.300) | 0.711 (0.455–1.108) |
| Adjusted OR (95% CI)a | 1.000 | 0.751 (0.522–1.078) | 0.747 (0.509–1.092) | 0.523 (0.324–0.841)e |
| Adjusted OR (95% CI)b | 1.000 | 0.641 (0.437–0.937)d | 0.548 (0.350–0.855)e | 0.329 (0.182–0.591)f |
| Adjusted OR (95% CI)c | 1.000 | 0.670 (0.452–0.989)d | 0.569 (0.360–0.896)d | 0.349 (0.190–0.637)f |
|
|
||||
| Women | ||||
| Number of subjects | 154 | 571 | 568 | 213 |
| Positive HGI | ||||
| Unadjusted OR | 1.000 | 1.039 (0.722–1.489) | 0.979 (0.680–1.402) | 0.991 (0.650–1.508) |
| Adjusted OR (95% CI)a | 1.000 | 1.060 (0.736–1.521) | 1.036 (0.716–1.493) | 1.083 (0.703–1.666) |
| Adjusted OR (95% CI)b | 1.000 | 0.970 (0.666–1.407) | 0.860 (0.569–1.295) | 0.797 (0.470–1.348) |
| Adjusted OR (95% CI)c | 1.000 | 0.954 (0.650–1.394) | 0.866 (0.569–1.311) | 0.826 (0.482–1.410) |
HGI, hemoglobin glycation index; Q, quartile (Q1 was the lowest quartile); OR, odds ratio; CI, confidence interval.
c As in b, and additionally adjusted for systolic blood pressure, exercise status, smoking status, hypoglycemic agent usage (insulin, sulfonylurea, biguanide, alpha-glucosidase inhibitor, glitazone, dipeptidyl peptidase-4 inhibitor, or sodium-glucose transport protein-2 inhibitor), high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, and statin usage;
Table 4
| Variable | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| Men, HGI range | 68–81 | 82–86 | 87–91 | 92–107 |
| Number of subjects | 343 | 414 | 389 | 423 |
| Positive HGI | ||||
| Unadjusted OR | 1.000 | 0.974 (0.731–1.297) | 1.256 (0.939–1.681) | 1.163 (0.875–1.548) |
| Adjusted OR (95% CI)a | 1.000 | 0.968 (0.726–1.290) | 1.246 (0.931–1.668) | 1.141 (0.856–1.520) |
| Adjusted OR (95% CI)b | 1.000 | 1.014 (0.740–1.390) | 1.349 (0.936–1.945) | 1.296 (0.823–2.044) |
| Adjusted OR (95% CI)c | 1.000 | 0.966 (0.697–1.338) | 1.264 (0.865–1.849) | 1.203 (0.749–1.935) |
|
|
||||
| Women, HGI range | 60–75 | 76–80 | 81–85 | 86–103 |
| Number | 361 | 382 | 338 | 425 |
| Positive HGI | ||||
| Unadjusted OR | 1.000 | 1.133 (0.848–1.513) | 1.277 (0.947–1.725) | 1.547 (1.163–2.062)e |
| Adjusted OR (95% CI)a | 1.000 | 1.113 (0.832–1.488) | 1.248 (0.923–1.687) | 1.503 (1.127–2.006)e |
| Adjusted OR (95% CI)b | 1.000 | 1.162 (0.852–1.585) | 1.345 (0.943–1.921) | 1.709 (1.108–2.642)d |
| Adjusted OR (95% CI)c | 1.000 | 1.117 (0.811–1.539) | 1.313 (0.908–1.901) | 1.669 (1.062–2.630)d |
HGI, hemoglobin glycation index; Q, quartile (Q1 was the lowest quartile); OR, odds ratio; CI, confidence interval.
c As in b, and additionally adjusted for systolic blood pressure, exercise status, smoking status, hypoglycemic agent usage (insulin, sulfonylurea, biguanide, alpha-glucosidase inhibitor, glitazone, dipeptidyl peptidase-4 inhibitor, or sodium-glucose transport protein-2 inhibitor), high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, and statin usage;
The risk of carotid plaque according to thigh circumference and waist circumference
Table 5
| Variable | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| Men | ||||
| Number of subjects | 147 | 710 | 547 | 171 |
| Plaque (+) | ||||
| Unadjusted OR | 1.000 | 0.664 (0.441–0.983)d | 0.460 (0.303–0.685)f | 0.215 (0.131–0.345)f |
| Adjusted OR (95% CI)a | 1.000 | 0.916 (0.593–1.394) | 0.899 (0.574–1.389) | 0.634 (0.370–1.076) |
| Adjusted OR (95% CI)b | 1.000 | 0.708 (0.448–1.103) | 0.548 (0.325–0.911)d | 0.304 (0.156–0.586)f |
| Adjusted OR (95% CI)c | 1.000 | 0.755 (0.473–1.190) | 0.587 (0.345–0.988)d | 0.352 (0.178–0.689)e |
|
|
||||
| Women | ||||
| Number of subjects | 154 | 572 | 569 | 217 |
| Plaque (+) | ||||
| Unadjusted OR | 1.000 | 0.801 (0.550–1.156) | 0.631 (0.434–0.910)d | 0.478 (0.311–0.730)f |
| Adjusted OR (95% CI)a | 1.000 | 0.884 (0.596–1.301) | 0.860 (0.579–1.269) | 0.767 (0.485–1.207) |
| Adjusted OR (95% CI)b | 1.000 | 0.818 (0.546–1.218) | 0.730 (0.469–1.129) | 0.587 (0.335–1.021) |
| Adjusted OR (95% CI)c | 1.000 | 0.734 (0.483–1.106) | 0.714 (0.454–1.114) | 0.590 (0.332–1.041) |
c As in b, and additionally adjusted for systolic blood pressure, exercise status, smoking status, hypoglycemic agent usage (insulin, sulfonylurea, biguanide, alpha-glucosidase inhibitor, glitazone, dipeptidyl peptidase-4 inhibitor, or sodium-glucose transport protein-2 inhibitor), high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, and statin usage;
Table 6
| Variable | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| Men | ||||
| Number of subjects | 343 | 414 | 389 | 423 |
| Plaque (+) | ||||
| Unadjusted OR | 1.000 | 0.950 (0.709–1.273) | 0.949 (0.705–1.276) | 1.067 (0.796–1.429) |
| Adjusted OR (95% CI)a | 1.000 | 0.981 (0.715–1.345) | 0.998 (0.725–1.373) | 1.207 (0.878–1.658) |
| Adjusted OR (95% CI)b | 1.000 | 0.877 (0.618–1.243) | 0.826 (0.55–1.239) | 0.894 (0.538–1.485) |
| Adjusted OR (95% CI)c | 1.000 | 0.824 (0.572–1.185) | 0.734 (0.479–1.122) | 0.831 (0.487–1.415) |
|
|
||||
| Women | ||||
| Number of subjects | 361 | 382 | 338 | 425 |
| Plaque (+) | ||||
| Unadjusted OR | 1.000 | 1.321 (0.989–1.765) | 1.234 (0.916–1.663) | 1.459 (1.1–1.938)e |
| Adjusted OR (95% CI)a | 1.000 | 1.185 (0.874–1.607) | 1.055 (0.77–1.444) | 1.217 (0.903–1.64) |
| Adjusted OR (95% CI)b | 1.000 | 1.209 (0.873–1.673) | 1.091 (0.752–1.582) | 1.290 (0.821–2.027) |
| Adjusted OR (95% CI)c | 1.000 | 1.047 (0.745–1.469) | 0.939 (0.635–1.387) | 1.039 (0.645–1.675) |
c As in b, and additionally adjusted for systolic blood pressure, exercise status, smoking status, hypoglycemic agent usage (insulin, sulfonylurea, biguanide, alpha-glucosidase inhibitor, glitazone, dipeptidyl peptidase-4 inhibitor, or sodium-glucose transport protein-2 inhibitor), high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, and statin usage;




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download