1. Colao A, Grasso LFS, Giustina A, Melmed S, Chanson P, Pereira AM, et al. Acromegaly. Nat Rev Dis Primers. 2019; 5:20.

2. Hong JW, Ku CR, Kim SH, Lee EJ. Characteristics of acromegaly in Korea with a literature review. Endocrinol Metab (Seoul). 2013; 28:164–8.

3. Melmed S. Medical progress: acromegaly. N Engl J Med. 2006; 355:2558–73.
4. Dekkers OM, Biermasz NR, Pereira AM, Romijn JA, Vandenbroucke JP. Mortality in acromegaly: a metaanalysis. J Clin Endocrinol Metab. 2008; 93:61–7.

5. Holdaway IM. Excess mortality in acromegaly. Horm Res. 2007; 68(Suppl 5):166–72.

6. Mercado M, Gonzalez B, Vargas G, Ramirez C, de los Monteros AL, Sosa E, et al. Successful mortality reduction and control of comorbidities in patients with acromegaly followed at a highly specialized multidisciplinary clinic. J Clin Endocrinol Metab. 2014; 99:4438–46.

7. Bex M, Abs R, T’Sjoen G, Mockel J, Velkeniers B, Muermans K, et al. AcroBel: the Belgian registry on acromegaly: a survey of the ‘real-life’ outcome in 418 acromegalic subjects. Eur J Endocrinol. 2007; 157:399–409.
8. Burton T, Le Nestour E, Neary M, Ludlam WH. Incidence and prevalence of acromegaly in a large US health plan database. Pituitary. 2016; 19:262–7.

9. Caputo M, Ucciero A, Mele C, De Marchi L, Magnani C, Cena T, et al. Use of administrative health databases to estimate incidence and prevalence of acromegaly in Piedmont region, Italy. J Endocrinol Invest. 2019; 42:397–402.

10. Dal J, Feldt-Rasmussen U, Andersen M, Kristensen LO, Laurberg P, Pedersen L, et al. Acromegaly incidence, prevalence, complications and long-term prognosis: a nationwide cohort study. Eur J Endocrinol. 2016; 175:181–90.

11. Gruppetta M, Mercieca C, Vassallo J. Prevalence and incidence of pituitary adenomas: a population based study in Malta. Pituitary. 2013; 16:545–53.

12. Hoskuldsdottir GT, Fjalldal SB, Sigurjonsdottir HA. The incidence and prevalence of acromegaly, a nationwide study from 1955 through 2013. Pituitary. 2015; 18:803–7.

13. Kwon O, Song YD, Kim SY, Lee EJ. Rare Disease Study Group, Science and Research Committee, Korean Endocrine Society. Nationwide survey of acromegaly in South Korea. Clin Endocrinol (Oxf). 2013; 78:577–85.

14. Mestron A, Webb SM, Astorga R, Benito P, Catala M, Gaztambide S, et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol. 2004; 151:439–46.

15. Park KH, Lee EJ, Seo GH, Ku CR. Risk for acromegaly-related comorbidities by sex in Korean acromegaly. J Clin Endocrinol Metab. 2020; 105:dgz317.

16. Raappana A, Koivukangas J, Ebeling T, Pirila T. Incidence of pituitary adenomas in Northern Finland in 1992–2007. J Clin Endocrinol Metab. 2010; 95:4268–75.

17. Reincke M, Petersenn S, Buchfelder M, Gerbert B, Skrobek-Engel G, Franz H, et al. The German Acromegaly Registry: description of the database and initial results. Exp Clin Endocrinol Diabetes. 2006; 114:498–505.

18. Tjornstrand A, Gunnarsson K, Evert M, Holmberg E, Ragnarsson O, Rosen T, et al. The incidence rate of pituitary adenomas in Western Sweden for the period 2001–2011. Eur J Endocrinol. 2014; 171:519–26.
19. Portocarrero-Ortiz LA, Vergara-Lopez A, Vidrio-Velazquez M, Uribe-Diaz AM, Garcia-Dominguez A, Reza-Albarran AA, et al. The Mexican Acromegaly Registry: clinical and biochemical characteristics at diagnosis and therapeutic outcomes. J Clin Endocrinol Metab. 2016; 101:3997–4004.

20. Chin SO, Ku CR, Kim BJ, Kim SW, Park KH, Song KH, et al. Medical treatment with somatostatin analogues in acromegaly: position statement. Endocrinol Metab (Seoul). 2019; 34:53–62.

21. Lavrentaki A, Paluzzi A, Wass JA, Karavitaki N. Epidemiology of acromegaly: review of population studies. Pituitary. 2017; 20:4–9.

22. Gadelha MR. A paradigm shift in the medical treatment of acromegaly: from a ‘trial and error’ to a personalized therapeutic decision-making process. Clin Endocrinol (Oxf). 2015; 83:1–2.

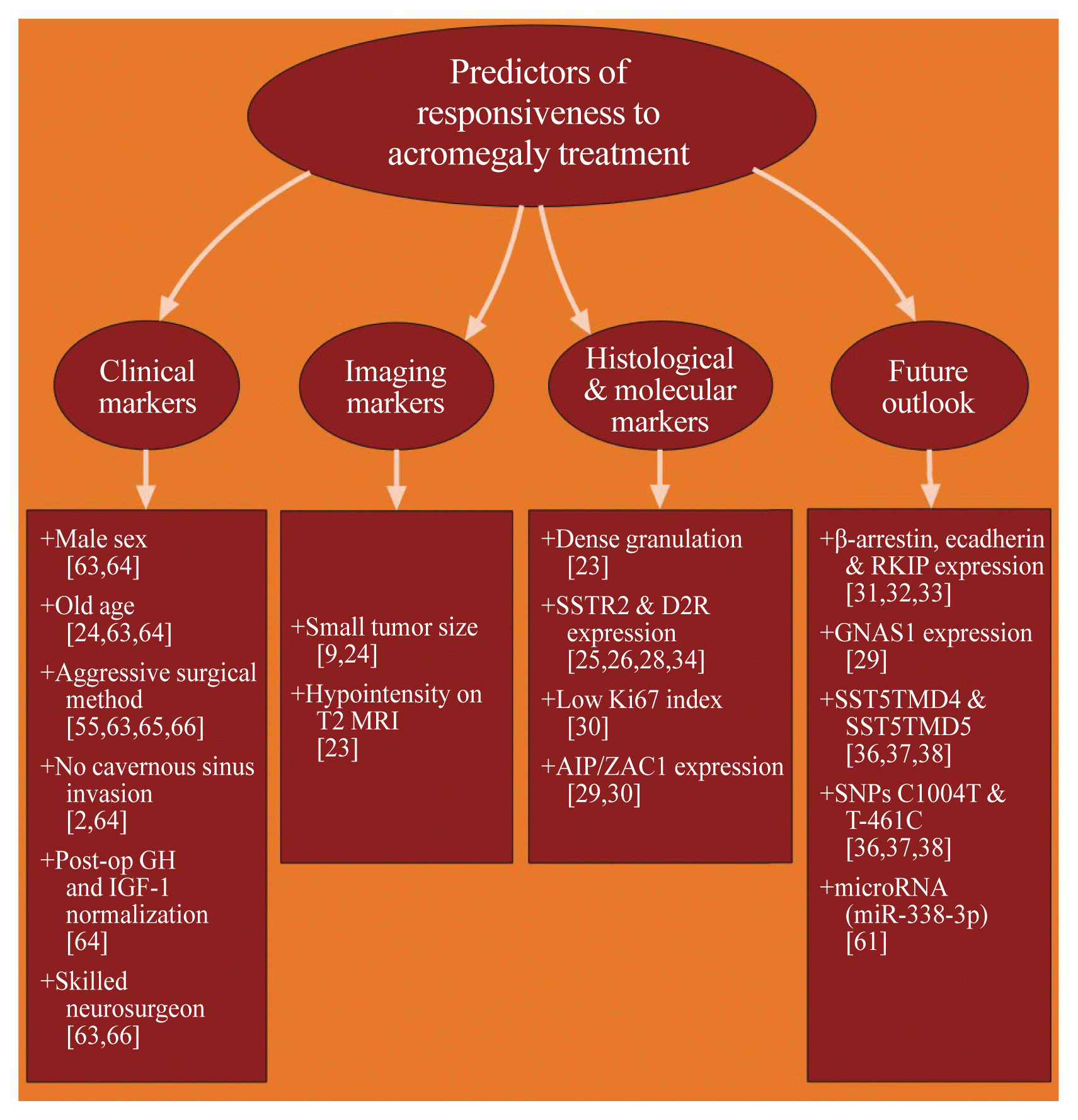
23. Heck A, Ringstad G, Fougner SL, Casar-Borota O, Nome T, Ramm-Pettersen J, et al. Intensity of pituitary adenoma on T2-weighted magnetic resonance imaging predicts the response to octreotide treatment in newly diagnosed acromegaly. Clin Endocrinol (Oxf). 2012; 77:72–8.

24. Petersenn S, Buchfelder M, Gerbert B, Franz H, Quabbe HJ, Schulte HM, et al. Age and sex as predictors of biochemical activity in acromegaly: analysis of 1485 patients from the German Acromegaly Register. Clin Endocrinol (Oxf). 2009; 71:400–5.

25. Casar-Borota O, Heck A, Schulz S, Nesland JM, Ramm-Pettersen J, Lekva T, et al. Expression of SSTR2a, but not of SSTRs 1, 3, or 5 in somatotroph adenomas assessed by monoclonal antibodies was reduced by octreotide and correlated with the acute and long-term effects of octreotide. J Clin Endocrinol Metab. 2013; 98:E1730–9.

26. Cuevas-Ramos D, Carmichael JD, Cooper O, Bonert VS, Gertych A, Mamelak AN, et al. A structural and functional acromegaly classification. J Clin Endocrinol Metab. 2015; 100:122–31.

27. Gillam MP, Ku CR, Lee YJ, Kim J, Kim SH, Lee SJ, et al. Somatotroph-specific Aip-deficient mice display pretumorigenic alterations in cell-cycle signaling. J Endocr Soc. 2017; 1:78–95.

28. Liu W, Xie L, He M, Shen M, Zhu J, Yang Y, et al. Expression of somatostatin receptor 2 in somatotropinoma correlated with the short-term efficacy of somatostatin analogues. Int J Endocrinol. 2017; 2017:9606985.

29. Rostomyan L, Beckers A. Screening for genetic causes of growth hormone hypersecretion. Growth Horm IGF Res. 2016; 30–31:52–57.

30. Fernandez-Rodriguez E, Casanueva FF, Bernabeu I. Update on prognostic factors in acromegaly: is a risk score possible? Pituitary. 2015; 18:431–40.

31. Fougner SL, Bollerslev J, Latif F, Hald JK, Lund T, Ramm-Pettersen J, et al. Low levels of raf kinase inhibitory protein in growth hormone-secreting pituitary adenomas correlate with poor response to octreotide treatment. J Clin Endocrinol Metab. 2008; 93:1211–6.

32. Fougner SL, Lekva T, Borota OC, Hald JK, Bollerslev J, Berg JP. The expression of E-cadherin in somatotroph pituitary adenomas is related to tumor size, invasiveness, and somatostatin analog response. J Clin Endocrinol Metab. 2010; 95:2334–42.

33. Gatto F, Biermasz NR, Feelders RA, Kros JM, Dogan F, van der Lely AJ, et al. Low beta-arrestin expression correlates with the responsiveness to long-term somatostatin analog treatment in acromegaly. Eur J Endocrinol. 2016; 174:651–62.

34. Neto LV, de Machado OE, Luque RM, Taboada GF, Marcondes JB, Chimelli LM, et al. Expression analysis of dopamine receptor subtypes in normal human pituitaries, nonfunctioning pituitary adenomas and somatotropinomas, and the association between dopamine and somatostatin receptors with clinical response to octreotide-LAR in acromegaly. J Clin Endocrinol Metab. 2009; 94:1931–7.

35. Shen M, Wang M, He W, He M, Qiao N, Ma Z, et al. Impact of long-acting somatostatin analogues on glucose metabolism in acromegaly: a hospital-based study. Int J Endocrinol. 2018; 2018:3015854.

36. Duran-Prado M, Gahete MD, Martinez-Fuentes AJ, Luque RM, Quintero A, Webb SM, et al. Identification and characterization of two novel truncated but functional isoforms of the somatostatin receptor subtype 5 differentially present in pituitary tumors. J Clin Endocrinol Metab. 2009; 94:2634–43.
37. Filopanti M, Ronchi C, Ballare E, Bondioni S, Lania AG, Losa M, et al. Analysis of somatostatin receptors 2 and 5 polymorphisms in patients with acromegaly. J Clin Endocrinol Metab. 2005; 90:4824–8.

38. Lania A, Mantovani G, Spada A. Genetic abnormalities of somatostatin receptors in pituitary tumors. Mol Cell Endocrinol. 2008; 286:180–6.

39. Khamseh ME, Mohajeri Tehrani MR, Mousavi Z, Malek M, Imani M, Hoshangian Tehrani N, et al. Iran Pituitary Tumor Registry: description of the program and initial results. Arch Iran Med. 2017; 20:746–51.
40. Tseng FY, Huang TS, Lin JD, Chen ST, Wang PW, Chen JF, et al. A registry of acromegaly patients and one year following up in Taiwan. J Formos Med Assoc. 2019; 118:1430–7.

41. Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab. 2006; 91:4769–75.

42. Fernandez A, Karavitaki N, Wass JA. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf). 2010; 72:377–82.

43. Maione L, Brue T, Beckers A, Delemer B, Petrossians P, Borson-Chazot F, et al. Changes in the management and comorbidities of acromegaly over three decades: the French Acromegaly Registry. Eur J Endocrinol. 2017; 176:645–55.

44. Petrossians P, Daly AF, Natchev E, Maione L, Blijdorp K, Sahnoun-Fathallah M, et al. Acromegaly at diagnosis in 3173 patients from the Liège Acromegaly Survey (LAS) Database. Endocr Relat Cancer. 2017; 24:505–18.

45. Vallette S, Ezzat S, Chik C, Ur E, Imran SA, Van Uum S, et al. Emerging trends in the diagnosis and treatment of acromegaly in Canada. Clin Endocrinol (Oxf). 2013; 79:79–85.

46. Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab. 2000; 85:526–9.

47. Chen CJ, Ironside N, Pomeraniec IJ, Chivukula S, Buell TJ, Ding D, et al. Microsurgical versus endoscopic transsphenoidal resection for acromegaly: a systematic review of outcomes and complications. Acta Neurochir (Wien). 2017; 159:2193–207.

48. Ding D, Mehta GU, Patibandla MR, Lee CC, Liscak R, Kano H, et al. Stereotactic radiosurgery for acromegaly: an international multicenter retrospective cohort study. Neurosurgery. 2019; 84:717–25.

49. Kim EH, Oh MC, Chang JH, Moon JH, Ku CR, Chang WS, et al. Postoperative gamma knife radiosurgery for cavernous sinus-invading growth hormone-secreting pituitary adenomas. World Neurosurg. 2018; 110:e534–45.

50. Lee CC, Vance ML, Xu Z, Yen CP, Schlesinger D, Dodson B, et al. Stereotactic radiosurgery for acromegaly. J Clin Endocrinol Metab. 2014; 99:1273–81.

51. Howlett TA, Willis D, Walker G, Wass JA, Trainer PJ. UK Acromegaly Register Study Group (UKAR-3). Control of growth hormone and IGF1 in patients with acromegaly in the UK: responses to medical treatment with somatostatin analogues and dopamine agonists. Clin Endocrinol (Oxf). 2013; 79:689–99.

52. Fleseriu M, Hoffman AR, Katznelson L. AACE Neuroendocrine and Pituitary Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: management of acromegaly patients: what is the role of pre-operative medical therapy? Endocr Pract. 2015; 21:668–73.

53. Alvarez-Escola C, Cardenas-Salas J. Active postoperative acromegaly: sustained remission after discontinuation of somatostatin analogues. Endocrinol Diabetes Metab Case Rep. 2016; 2016:16-0092.

54. Lim DS, Fleseriu M. The role of combination medical therapy in the treatment of acromegaly. Pituitary. 2017; 20:136–48.

55. Kim EH, Ku CR, Lee EJ, Kim SH. Extracapsular en bloc resection in pituitary adenoma surgery. Pituitary. 2015; 18:397–404.

56. Strasburger CJ, Mattsson A, Wilton P, Aydin F, Hey-Hadavi J, Biller BMK. Increasing frequency of combination medical therapy in the treatment of acromegaly with the GH receptor antagonist pegvisomant. Eur J Endocrinol. 2018; 178:321–9.

57. Neggers SJ, Franck SE, de Rooij FW, Dallenga AH, Poublon RM, Feelders RA, et al. Long-term efficacy and safety of pegvisomant in combination with long-acting somatostatin analogs in acromegaly. J Clin Endocrinol Metab. 2014; 99:3644–52.

58. Lin AL, Sum MW, DeAngelis LM. Is there a role for early chemotherapy in the management of pituitary adenomas? Neuro Oncol. 2016; 18:1350–6.

59. Kim JM, Lee YH, Ku CR, Lee EJ. The cyclic pentapeptide d-Arg3FC131, a CXCR4 antagonist, induces apoptosis of somatotrope tumor and inhibits tumor growth in nude mice. Endocrinology. 2011; 152:536–44.

60. Lee YH, Noh TW, Lee MK, Jameson JL, Lee EJ. Absence of activating mutations of CXCR4 in pituitary tumours. Clin Endocrinol (Oxf). 2010; 72:209–13.
61. Lee YJ, Cho JM, Moon JH, Ku CR, Kim J, Kim SH, et al. Increased miR-338–3p expression correlates with invasiveness of GH-producing pituitary adenomas. Endocrine. 2017; 58:184–9.

62. Lee EJ, Jameson JL. Gene therapy of pituitary diseases. J Endocrinol. 2005; 185:353–62.

63. Ku CR, Kim EH, Oh MC, Lee EJ, Kim SH. Surgical and endocrinological outcomes in the treatment of growth hormone-secreting pituitary adenomas according to the shift of surgical paradigm. Neurosurgery. 2012; 71(2 Suppl Operative):ons192–203.

64. Park SH, Ku CR, Moon JH, Kim EH, Kim SH, Lee EJ. Age- and sex-specific differences as predictors of surgical remission among patients with acromegaly. J Clin Endocrinol Metab. 2018; 103:909–16.

65. Lee EJ, Ahn JY, Noh T, Kim SH, Kim TS, Kim SH. Tumor tissue identification in the pseudocapsule of pituitary adenoma: should the pseudocapsule be removed for total resection of pituitary adenoma? Neurosurgery. 2009; 64(3 Suppl):ons62–9.

66. Park HH, Kim EH, Ku CR, Lee EJ, Kim SH. Outcomes of aggressive surgical resection in growth hormone-secreting pituitary adenomas with cavernous sinus invasion. World Neurosurg. 2018; 117:e280–9.

67. Kim EH, Oh MC, Lee EJ, Kim SH. Predicting long-term remission by measuring immediate postoperative growth hormone levels and oral glucose tolerance test in acromegaly. Neurosurgery. 2012; 70:1106–13.

68. Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014; 99:3933–51.

69. Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, et al. A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010; 95:3141–8.

70. Ku CR, Choe EY, Hong JW, Kim EH, Park SH, Kim SH, et al. No differences in metabolic outcomes between nadir GH 0.4 and 1.0 ng/mL during OGTT in surgically cured acromegalic patients (observational study). Medicine (Baltimore). 2016; 95:e3808.

71. Ku CR, Hong JW, Kim EH, Kim SH, Lee EJ. Clinical predictors of GH deficiency in surgically cured acromegalic patients. Eur J Endocrinol. 2014; 171:379–87.

72. Dutta P, Mahendran B, Reddy KS, Ahluwalia J, Vaiphei K, Kochhar RK, et al. Short-term efficacy of recombinant human GH therapy in cured acromegaly patients with GH deficiency: a single-center experience. Endocr Connect. 2015; 4:65–75.

73. Kim Y, Hong JW, Chung YS, Kim SW, Cho YW, Kim JH, et al. Efficacy and safety of sustained-release recombinant human growth hormone in Korean adults with growth hormone deficiency. Yonsei Med J. 2014; 55:1042–8.

74. Tritos NA, Johannsson G, Korbonits M, Miller KK, Feldt-Rasmussen U, Yuen KC, et al. Effects of long-term growth hormone replacement in adults with growth hormone deficiency following cure of acromegaly: a KIMS analysis. J Clin Endocrinol Metab. 2014; 99:2018–29.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download