A 33-year-old man received first decitabine chemotherapy for MDS, refractory anemia with excess blast type 1. He had received itraconazole (100 mg twice a day) for prophylaxis. Two weeks later, he visited the emergency room because of fever. On examination, vital signs were blood pressure 100/60 mmHg, temperature 36.9°C, heart rate 144/min, and respiratory rate 16/min. White blood cell (WBC) count was 1,030/mm
3 with 31% neutrophils, hemoglobin 7.0 g/dL, platelet count 3,000/mm
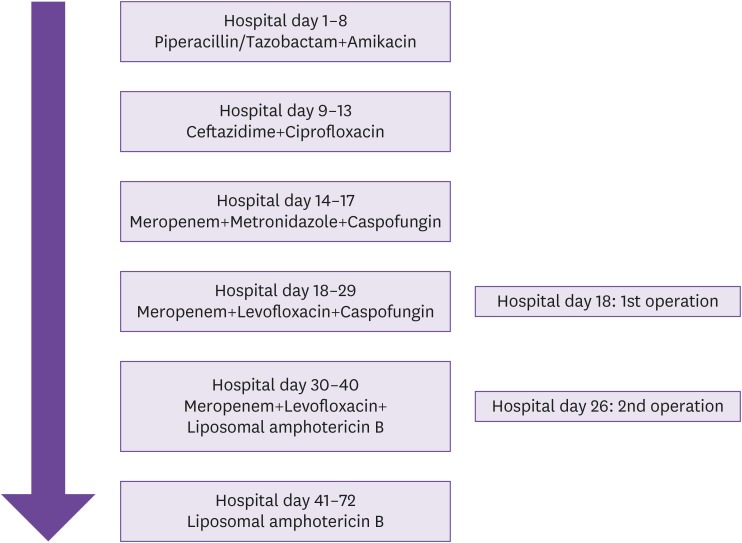
3, total bilirubin 2.11 mg/dL, and C-reactive protein level 3.33 mg/dL. Serum galactomannan antigen was negative. An abdominal radiograph showed mild non-specific paralytic ileus, but there was no pain and tenderness on any abdominal point. There were no abnormal findings on chest radiograph and urinalysis. He received granulocyte colony-stimulating factor (G-CSF), piperacillin/sulbactam (4.5 g four times a day), and amikacin (7.5 mg/kg twice a day) for febrile neutropenia (
Fig. 1). On hospital day 9 (chemotherapy day 27), he complained of whole abdominal cramping pain. There was tenderness as well as rebound tenderness on the whole abdomen. On examination, vital signs were blood pressure 120/80 mmHg, temperature 37.8°C, heart rate 90/min, and respiratory rate 20/min. WBC count was 420/mm
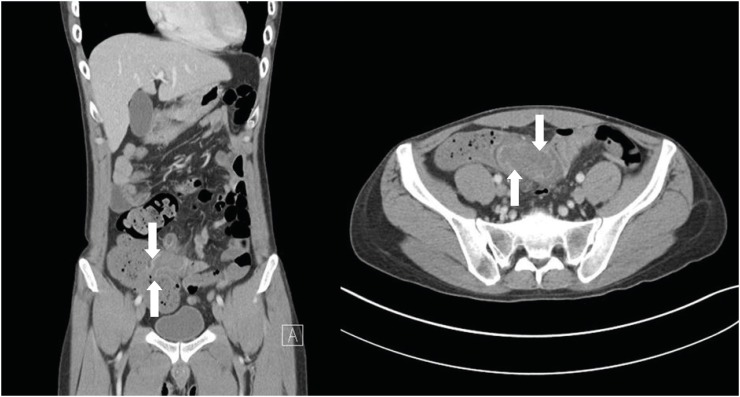
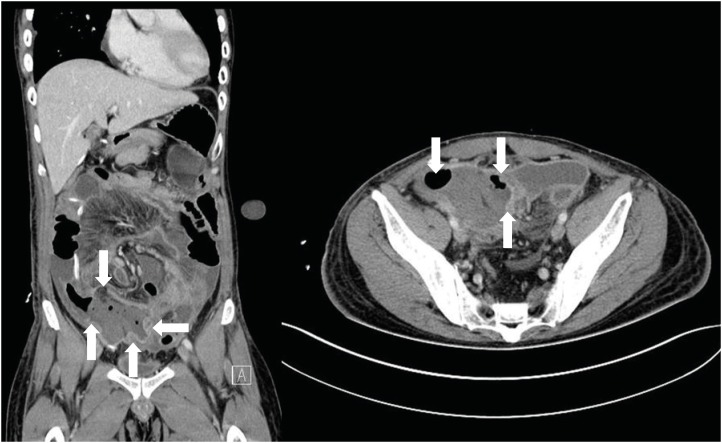
3 with 4.7% neutrophils. Computed tomography (CT) of the abdomen revealed abnormal wall thickening and decreased density of the ileocecal valve and terminal ileum, suggesting neutropenic enterocolitis (
Fig. 2). Supportive treatment including nasogastric tube drainage, G-CSF, ceftazidime (2 g three times a day), and ciprofloxacin (400 mg twice a day) was started due to neutropenia (WBC count 420/mm
3) and thrombocytopenia (platelet count 3,000/mm
3) (
Fig. 1). Patient was broadened the spectrum of antimicrobial agents to meropenem (1 g three times a day) and metronidazole (500 mg three times a day), and an antifungal agent (caspofungin, 50 mg once daily) (
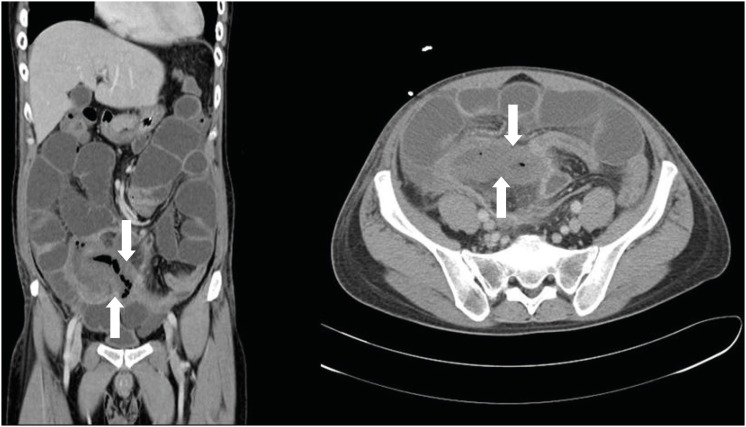
Fig. 1). Symptoms and signs including fever, abdominal pain, and tenderness on the whole abdomen did not improve. Another abdominal CT scan was done on hospital day 18, which revealed bowel perforation at ileocecal valve and terminal ileum with partial obstruction of bowel and peritonitis (
Fig. 3). Emergent exploratory laparotomy was performed. Gross finding showed whole small bowel dilatation, ileocecal region perforation, and ascending colon inflammation. Small bowel resection at ileocecal lesion was performed. After surgery, the patient received G-CSF, meropenem, levofloxacin (750 mg once daily), and caspofungin until the biopsy was confirmed (
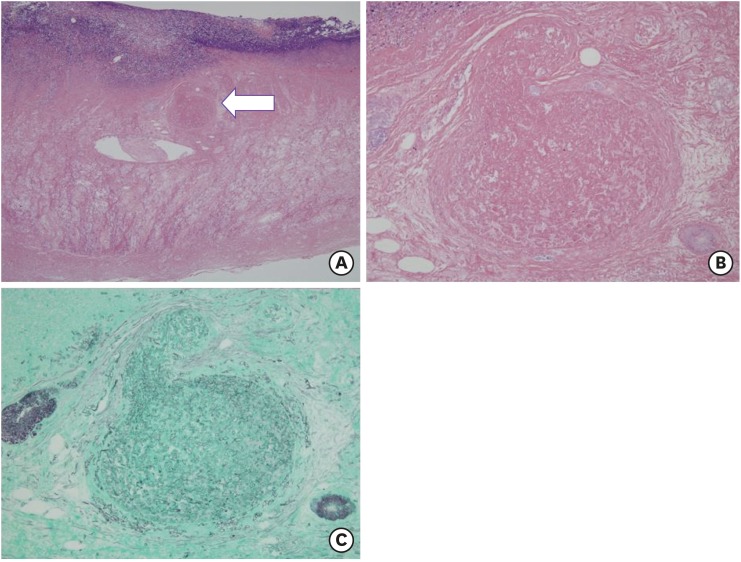
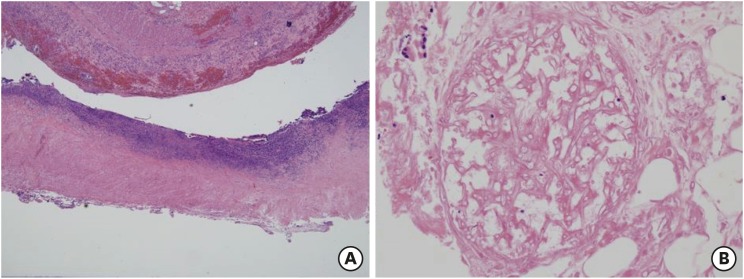
Fig. 1). Microscopic finding showed hemorrhagic infarction associated with invasive fungal infection with right angle hyphae corresponding with mucormycosis (
Fig. 4). After the histopathologic confirmation, caspofungin was switched to liposomal amphotericin B (5 mg/kg/day) (
Fig. 1). On hospital day 21, he still complained of whole abdominal pain, although fever and the inflammation marker were resolved and neutrophil counts had recovered. A second follow-up abdominal CT scan performed on hospital day 21 showed bowel perforation, gangrenous change at ileocecal valve, terminal ileum, and multifocal complicated fluid collection in the peritoneum due to sealed-off perforation (
Fig. 5). Galactomanan or 1,3-beta-D glucan level was not examined that day. On hospital day 26, exploratory laparotomy was performed again, which revealed perforation of the previous anastomosis site. Segmentectomy of the small bowel perforation, mobilization of ascending colon, and ascending colon transection were performed. Microscopic histopathologic findings showed hemorrhagic infarction and fungal hyphae at resected small bowel and resected ascending colon (
Fig. 6). He received liposomal amphotericin B for 6 weeks and was finally discharged from hospital with full recovery of general condition. Another abdominal CT scan confirmed the absence of perforation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download