Introduction
Severe trauma causes tissue damage, collapse of the skin barrier, and alterations in the immune system. As a result, patients with severe trauma are vulnerable to bacterial invasion and infection [
12]. In particular, methicillin-resistant
Staphylococcus aureus is a major pathogen in intensive care units (ICUs) and patients with severe trauma can easily contract
S. aureus infection [
3].
Vancomycin is the first-line antibiotic used in the treatment of methicillin-resistant
S. aureus (MRSA) infection [
4]. Vancomycin is an area under the concentration curve-dependent antibiotic, and suboptimal serum concentrations of this drug lead to treatment failure and the development of resistance [
56]. Thus, serum vancomycin concentrations must be measured in patients with severe trauma by therapeutic drug monitoring (TDM) [
7]. In a 1,084-bed tertiary hospital which this study had done with a trauma ICU (TICU), suboptimal serum vancomycin concentrations were more frequently observed in severe trauma patients than medical intensive care unit (MICU) patients.
Vancomycin clearance (
CLvan) is influenced by many factors. A patient's renal function and use of continuous renal replacement therapy (CRRT) have a decisive effect on
CLvan. Capillary fluid leakage leads to changes in the volume of distribution (
Vd) and
CLvan [
8]. Intensive fluid therapy for critically ill patients causes pharmacokinetic alterations due to the hydrophilicity of vancomycin [
5]. The simultaneous use of other drugs influencing renal function and the use of vasoactive drugs affect
CLvan [
59].
In this study, the factors affecting vancomycin levels between multiple trauma patients in the TICU and MICU stratified by use of CRRT were compared. Furthermore, the influence of these factors on CLvan was statistically analyzed.
Go to :

Discussion
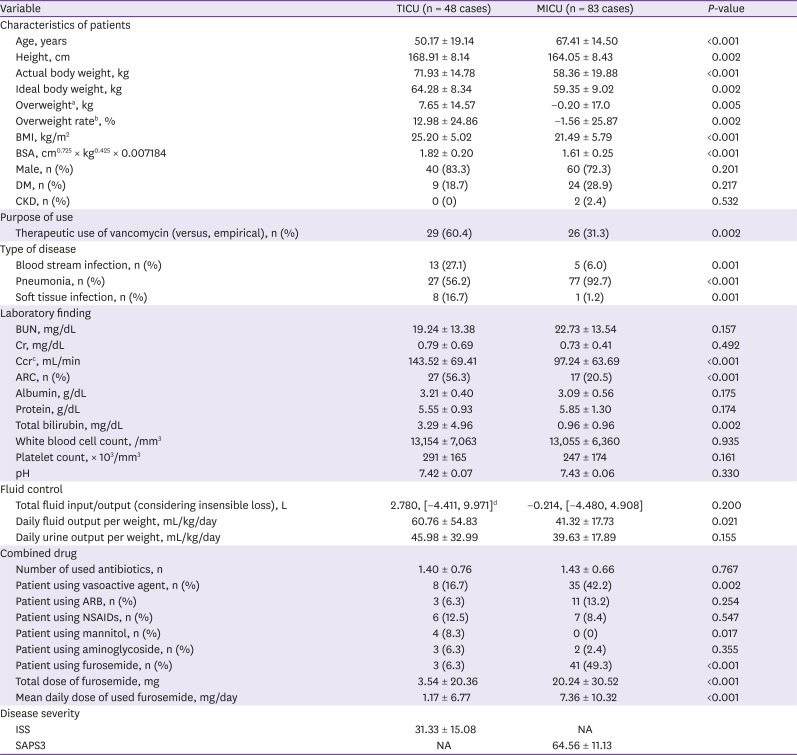
In this study, multiple trauma patients in the TICU were compared to patients in the MICU for the identification of the factors associated with CLvan. The patients' factors that significantly differed between the TICU and MICU patients were age, height, actual body weight, IBW, overweight, BMI, BSA, therapeutic use of vancomycin (vs. empiric use), site of infection, serum Ccr, rate of ARC, serum total bilirubin, one-day fluid output per weight, use of vasoactive agents, use of mannitol, use of furosemide, and furosemide dose.
Multiple trauma patients develop immune system alterations through tissue damage, resulting in capillary leakage and peripheral fluid retention [
1]. Such patients should undergo intensive fluid therapy for hemodynamic stabilization. These processes affect drug pharmacokinetics, augment renal drug elimination, increase the
Vd, and decrease serum drug concentrations [
8916]. Weight gain occurs because of traumatic damage and massive fluid therapy, thus reflecting the degree of damage and treatment intensity. It is expected that drug clearance increases with weight gain.
In this study, the largest differences between the two ICU patient groups were observed in the weight indices. The patients in the TICU were heavier and taller and had a higher IBW and rate of overweight than those in the MICU. The overweight rate was found to be 12.98 ± 24.8% in patients in the TICU undergoing high-intensity therapy and with a high degree of tissue damage. The actual body weight of the MICU patients was lower than their IBW; this may be a result of the long-term strict management of pulmonary edema which required negative fluid balance.
Serum Ccr is measured using the Cockcroft-Gault equation, which includes serum creatinine, age, and actual body weight. The serum Ccr values in this study were markedly different between the TICU and MICU patients (143.52 ± 69.41 mL/min vs. 97.24 ± 63.69 mL/min; P < 0.001), the rate of ARC was higher in TICU patients than MICU patients (56.3% vs. 20.5%; P < 0.001); however, the serum creatinine level did not differ (0.79 ± 0.69 mg/dL vs. 0.73 ± 0.41 mg/dL; P = 0.492). It is thought that ARC originate from age and the actual body weight of patients.
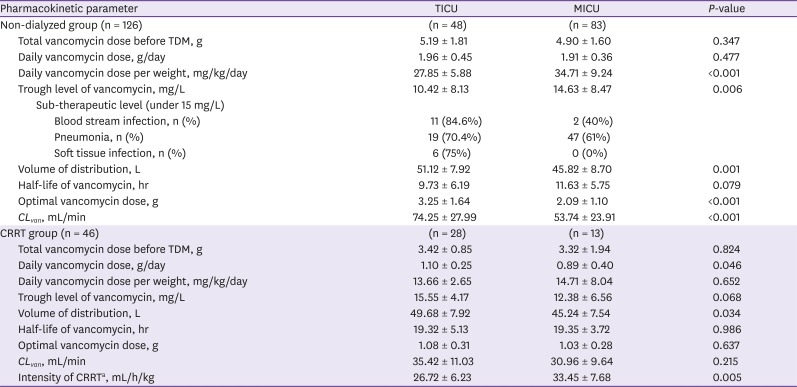
Table 2 shows the pharmacokinetics of vancomycin in the two groups according to the use of CRRT. The one-day vancomycin dose in the non-dialyzed group was not different between the two ICU group patients, and the actual body weight was higher in the TICU patients. As a result, the one-day vancomycin dose per weight is expected to be lower in TICU patients than MICU patients. The vancomycin dose was nearly 2 g/day in both ICUs, resulting from the consideration of IBW. Heavier TICU patients are administered a relatively lower dose, while the
CLvan is higher in TICU patients; therefore, the serum vancomycin peak and trough levels are lower in TICU patients than MICU patients. The
CLvan in the CRRT group was not different between the patients in the two ICUs. In the CRRT group, hemodiafiltration is the only method used for vancomycin elimination, so
CLvan is not affected by other patients' variables. Thus,
CLvan should not be considered in the determination of the vancomycin dose for patients in either ICU type.
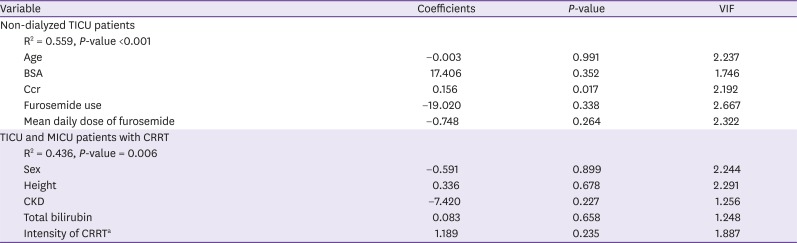
Table 3 shows the results of the multiple linear regression, in which correlations were observed between patient factors and
CLvan. In the non-dialyzed TICU group, the only factor showing a significant correlation was serum Ccr. The correlation between
CLvan and serum Ccr, as observed in our study, has been confirmed by studies including non-severe trauma patients and neurosurgical ICU patients [
1718].
As mentioned above, serum Ccr includes patient age, actual body weight, and serum creatinine concentrations. In our study, these three factors did not affect CLvan individually, but they were correlated with CLvan when considered simultaneously. Actual body weight showed the largest number of differences of the aforementioned three factors between the TICU and MICU patients. Thus, the actual body weight of patients should be considered in the determination of vancomycin dose.
In studies about ARC, the ARC associated with younger age, male gender, trauma, lower critical illness severity scores [
11]. In those studies, body weight was not so related with ARC. The reason is that there is no significant difference in weight from the control, thus weight indicators were not importantly analyzed [
192021]. In our study, the weight gain was important feature of TICU patients. Severe trauma patients required massive fluid therapy, thus patients with ARC should be taken care of weight.
Medellin-Garibay et al reported that the use of furosemide in trauma patients affects the pharmacokinetics of vancomycin, but our study showed no significant correlation with the use of furosemide [
18]. That study, however, included all types of trauma patients. However, in our study, we included severe trauma patients, who were hospitalized in the TICU; therefore, the number of patients using furosemide was small (n = 3, 6.3%) as was the furosemide dose (1.17 ± 6.77 mg/day). Further studies need to be conducted to clarify this point.
CRRT use may affect drug clearance. Drugs with a high molecular weight, such as vancomycin, are influenced more by CRRT intensity than the CRRT method [
2223]. In our study, many factors were analyzed for the determination of their association with
CLvan in the CRRT group, but none of the factors showed correlations, including the CRRT intensity (
Table 3). Previous studies differ from our study in that our participants included trauma patients. Patients in the TICU differ from those in the MICU based on the volume of fluid therapy and actual body weight, which affect drug pharmacokinetics; this may be the reason for the observed differences between our study and previous studies.
No difference in terms of
CLvan in the CRRT group was observed between the patients in the two ICUs (
Table 2), and there were no factors that affected
CLvan in both sets of patients (
Table 3). Therefore, vancomycin dosing can be performed using a fixed dose in patients undergoing CRRT.
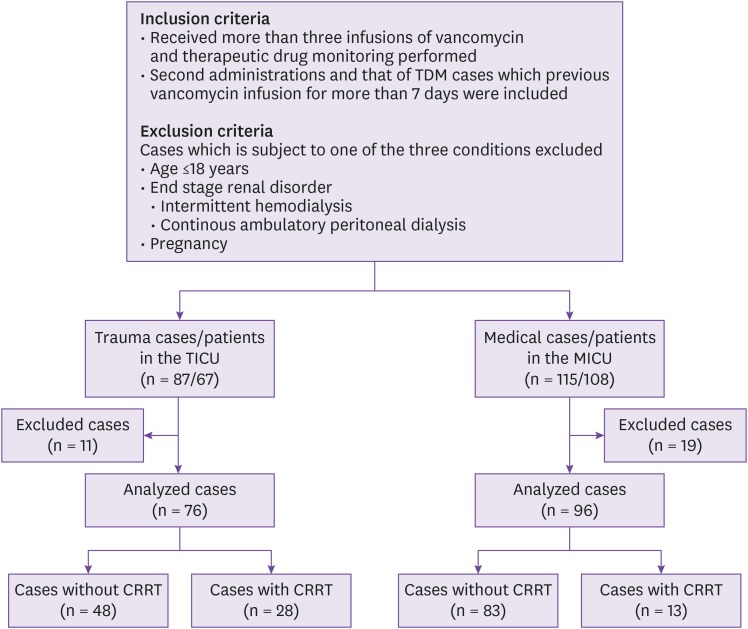
This study has several limitations that should be mentioned. First, the clinical characteristics including age and ideal body weight were different between two groups, so the control group may not be appropriate. Second, the sample size was relatively small for the identification of differences between the two groups. Third, this study was conducted at a single referral center in Far East Asia, so the results may not be applicable to other regions. Fourth, the number of patients using furosemide was small. Furosemide is an important drug affecting drug clearance. In this study, the influence of furosemide could not be determined.
The actual body weight of TICU patients with multiple trauma easily changes compared to other patients in the ICU because of intensive fluid therapy administration. And actual body weight would be an important factor of ARC. Therefore, the vancomycin dose should be controlled in non-dialyzed TICU patients, depending on the actual body weight changes. Further, therapeutic vancomycin levels should be monitored for the maintenance of optimal therapeutic levels of vancomycin. In the case of dialyzed patients in both ICUs, vancomycin can be infused with fixed doses regardless of patients' characteristics.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download