Abstract
Sepsis is one of the significant causes of morbidity and mortality. The burden caused by sepsis has continued to increase in recent years in the Korea, highlighting the urgent need for the implementation of strategies to improve sepsis treatment outcomes. We therefore designed a web-based sepsis registry system (“Korean Registry for Improving Sepsis Survival” [KISS]) protocol to be used in hospitals in the Korea for evaluation of the epidemiology and clinical characteristics of patients with sepsis, via an analysis of outcome predictors. The inclusion criteria of this registry are as follows: adult patients ≥18 years admitted to the participating hospitals who are diagnosed with sepsis or septic shock. Demographic and clinical information data of the patients will be collected from hospital medical records and will be recorded in a case report form, which will be entered into a web-based data management system. The analysis of the collected data will be performed as follows: (1) epidemiological and clinical characteristics of sepsis and septic shock, (2) application of sepsis bundles and antibiotic stewardship, and (3) audit and feedback. In conclusion, we aim to build the comprehensive web-based sepsis registry in the Korea through a nation-wide network of participating hospitals. Information collected and analyzed through the KISS can be used for further improvements in the clinical management of sepsis. Furthermore, the KISS will facilitate research leading to the formulation of public health policies regarding sepsis bundle and antibiotic stewardship strategies in the Korea.
Sepsis is one of the significant causes of morbidity and mortality; it stems from a systemic inflammatory reaction against infection manifesting in multi-organ failure syndrome. The burden of sepsis has been well described with the global annual incidence of sepsis and severe sepsis estimated at 31.5 million and 19.4 million, respectively, and 5.3 million deaths that suggest a severe sepsis-related mortality rate of around 30% [1]. Even in a high-income country such as the United States (US), sepsis continues to be one of the primary healthcare issues. A recent US government study revealed that sepsis-related hospitalization was 70% more expensive than average hospitalization, accounting for 8.2% of all Medicare costs in 2013 [2]. The estimated incidence of severe sepsis in the US is 751,000 cases with 215,000 deaths annually [3]. In the Korea, public awareness of sepsis has been reported to be lower than that of other common medical diseases such as acute myocardial infarction and stroke [4]. Furthermore, the sepsis-related mortality rate was estimated to be around 30% in the Korea [5], and the number of deaths from sepsis has recently increased at the national level (2011: 1,835 with a death rate of 3.7/100,000 persons; 2014: 2,503 with a death rate of 4.9/100,000; 2016: 3,596 with a death rate of 7.0/100,000; 2018: 4,656 with a death rate of 9.1/100,000) [6]. There is therefore an urgent need for the implementation of strategies to improve sepsis treatment outcomes in the Korea.
Early recognition and timely management of sepsis in a systemic manner are the critical components recommended by the Surviving Sepsis Guideline [7]. In particular, the early administration of antibiotics and fluids as part of the bundle approach, within 3 hours, has been emphasized owing to increased mortality risks associated with delayed 3-hour bundle sepsis care [8]. As increased compliance with the sepsis care bundle recommended by the Surviving Sepsis Guideline showed a reduction in hospital mortality [910], implementation of the standardized sepsis care bundle needs to be considered for optimal management of sepsis. In addition, our sepsis registry system may serve a valuable role in estimating the true incidence of sepsis as well as in monitoring the implementation of and adherence to standardized sepsis care bundles. A notable example is the New York State sepsis registry in the US [11]. Since 2014, hospitals in the state of New York are required to adopt a sepsis care bundle protocol including the early administration of antibiotics and to report on clinical data and compliance to the protocol. Increased compliance to the sepsis care bundle protocol accompanied by improved sepsis treatment outcomes in a cohort comprising New York State hospitals after 2014 was reported in a recent study [12], which suggests significant potential benefits associated with the implementation of such a protocol and registry system. There is, however, no nation-wide sepsis registry system in the Korea, in spite of the considerable incidence and disease burden caused by sepsis. Therefore, we designed a web-based sepsis registry system (“Korean Registry for Improving Sepsis Survival” [KISS]) protocol to be used in hospitals in the Korea to evaluate the epidemiological and clinical characteristics of patients with sepsis via an analysis of outcome predictors. The KISS protocol is described in the following.
The oversight committee for the KISS consists of members of the Korean Society of Infectious Diseases, who are responsible for the careful execution of the KISS project including the selection of the participating hospitals, the collection and analysis of the data, and the education of the research staff. The steering committee consists of members of the oversight committee, consultants, and computer development managers. The consultants consist of infectious disease physicians and experts in preventive medicine who will provide further advice on areas of improvement of the KISS project. The computer development managers are in charge of designing and maintaining the web-based registry data management system.
The KISS is designed as a prospective multicenter cohort enrolling adult patients with sepsis in the Korea. Patients will be screened for eligibility on a monthly basis by trained study nurses or physicians at the participating hospitals. Documentation using hospital medical records includes the following assessment factors recorded in a case report form (CRF): onset of sepsis, initial resuscitation, site of infection and appropriateness of antimicrobial treatment, and outcomes. Collected CRF data is submitted to the KISS via the designated data collection portal website for further analysis.
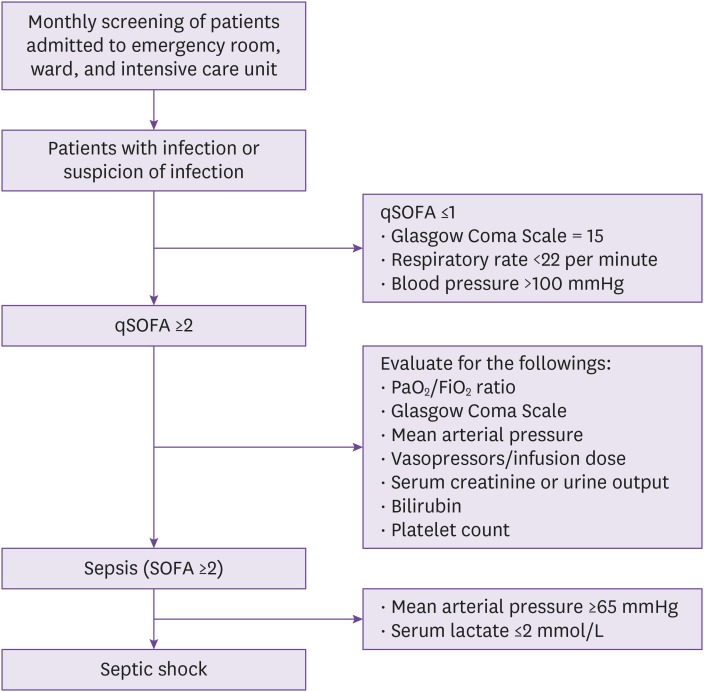
The multicenter hospital-based sepsis cohort will consist of approximately 30 hospitals from six major metropolitan cities and four provinces across the Korea. Patients with sepsis or septic shock will be identified via screenings by trained study nurses or physicians once a month at the participating hospitals across the Korea. The screened patients will be adult patients admitted to the hospital or emergency room who are initiated on intravenous antibiotics. The inclusion criteria will be as follows: adult patients ≥18 years admitted to the participating hospitals who are diagnosed with either (1) sepsis: a clinically suspected or microbiologically proven infection with presence of organ dysfunction, defined by a Sequential Organ Assessment (SOFA) score increase of more than 2, or (2) septic shock: the presence of sepsis with persistent hypotension despite volume resuscitation with the patient needing to be on a vasopressor to maintain a mean arterial pressure (MAP) ≥65 mmHg after appropriate fluid resuscitation and serum lactate levels >2 mmol/L according to the SEPSIS-3 criteria [13]. All patients diagnosed with sepsis or septic shock who are eligible according to these inclusion criteria will be enrolled into the KISS (Fig. 1).
Demographic and clinical data of the patients will be collected from hospital medical records and will be recorded in a CRF. The following variables will be listed: (1) demographic data including age, sex, admitting hospital department, and date of hospital admission; (2) initial symptoms and positive physical examination findings; (3) time to initiation of treatment against sepsis or septic shock; (4) information regarding the infection including site of infection, acquisition setting, category of sepsis, and Pitt bacteremia score; (5) comorbidities and presence of indwelling devices; (6) severity of sepsis or septic shock according to the Acute Physiology and Chronic Health Evaluation (APACHE) II/III score, the Simplified Acute Physiology Score (SAPS) II score, the Sequential Organ Failure Assessment (SOFA) score, and the Glasgow Coma Scale; (7) laboratory data and culture study data; (8) data on antibiotics including empiric antibiotics treatment and assessment of the appropriateness of antibiotic treatment according to the Surviving Sepsis Guideline [7] and culture data; (8) assessment of the Surviving Sepsis Campaign bundle (sepsis bundle) application; (9) additional treatments including initial fluid therapy, vasopressor, mechanical ventilation, and other therapies such as red blood cell transfusion, steroid, and intravenous immunoglobulin; (10) outcomes including duration of hospital stay, mortality (sepsis-related mortality and all-cause mortality), and discharge status. The sepsis cohort data CRF is shown in Supplementary Table 1.
The KISS system collects data through a web-based data management system, so that the research teams from the participating hospitals can report the collected data via a web browser. The selection of the registry and maintenance protocol of the KISS system will be monitored by the oversight committee. The collected data are securely deposited onto a data storage server with an available back-up system. The KISS system enables the analysis of collected data and the visualization in tables and figures; the submission of a proposal to the oversight committee is however required, and access to the data will only be granted after approval by the oversight committee.
The screening of patients and the data collection processes for the KISS will be initiated after each participating hospital has received approval from their institutional review board. The oversight committee for the KISS project will follow the recommendations from the institutional review board at each participating hospital and shall amend the protocol as needed.
The annual incidence of sepsis and septic shock at each participating hospital will be calculated and, on the basis of these data, the nation-wide annual incidence of sepsis and septic shock in the Korea will be estimated.
The epidemiological and clinical characteristics of sepsis and septic shock will be assessed using the following data: age, sex, route of hospital admission, duration of fever and presenting symptoms, site of infection, classification of sepsis and septic shock, Pitt bacteremia score, severity of sepsis (APACHE II score, SAPS II score, SOFA score, Modified Early Warning Score), laboratory data, identified pathogens from culture studies, use of vasopressors, use of other treatments, and appropriate application of the sepsis bundle. Risk factors associated with death from sepsis and septic shock will be assessed in a survival analysis. Furthermore, a comparison between the hospitals with high and low sepsis-related mortality will be performed to identify hospital- specific factors contributing to higher sepsis-related mortality. The role of antibiotic stewardship will be assessed according to the following factors: (1) empiric antibiotics: type and class, monotherapy vs. combination therapy, dosage and administration, and duration; (2) appropriateness of empiric antibiotics; (3) definitive antibiotics: type and class, monotherapy vs. combination therapy, dosage and administration, and duration; (4) de-escalation from empiric antibiotics to definitive antibiotics. In particular, the effect of the Surviving Sepsis Campaign bundle and antibiotic stewardship on 30-day mortality, multidrug-resistant bacterial pathogen infection rates, consumption of antibiotics, and durations of hospital stay will be assessed by case-control studies between the hospitals with and without implementation of sepsis bundle and antibiotic stewardship.
Monthly audits and the monitoring of data collected through the web-based data management system will be led by the oversight committee. The completeness and validity of the data will be assessed and query notifications will be sent to the participating hospitals if needed. Each participating hospital may check and update the collected data on the web site to help solve queries. Feedback from the participating hospitals will be sent to the oversight committee for further improvement of the KISS system.
Continuous variables will be compared using independent t-tests or the Mann-Whitney test. Categorical variables will be analyzed using Pearson's chi-square test or Fisher's exact test. Variables with a P-value <0.1 in the comparison analysis will be included in a multiple logistic regression to determine risk factors. Kaplan-Meier curves and Cox proportional hazard models will be used for survival analyses. Propensity scores will be constructed to ensure balance in baseline characteristics between the hospitals with and without the sepsis bundle and antibiotic stewardship in the case-control studies. The variables included in the propensity models will be epidemiological and clinical factors. All tests will be two-sided, and a P-value <0.05 will be considered statistically significant. Analyses will be performed using Stata version 14 (StataCorp LP, College Station, Texas, USA) and SPSS version 24.0 (IBM SPSS Inc., Chicago, IL, USA).
The KISS is the first prospective multicenter cohort registry using a web-based data collection system to offer advanced assessment of adult patients with sepsis in the Korea in the followings: (1) clinical epidemiology, management including implementation of sepsis bundle, and primary outcomes such as death, and (2) secondary outcomes measuring the impact of appropriate use of antibiotics and the role of antibiotic stewardship. With this comprehensive evaluation of adult patients with sepsis through the collection of clinical data via a web browser, we expect the KISS to offer valuable information on the current clinical epidemiological approach to sepsis in real-world settings in the Korea. Although a previous study [14] reported mortality and performance rates for individual sepsis bundle elements (including lactate measurements, administration of fluids and antibiotics, and central pressure measurements) among adult patients with septic shock in the Korea, the data was based on a registry from a relatively smaller number of hospitals (n = 10), which limits the study's informative value. The KISS registry will be based on a more significant number of hospitals (n = 30) across the Korea, and is thus expected to provide a more accurate assessment of the clinical epidemiology of sepsis in the Korea.
The KISS will help identify compliance with and variation in the application of sepsis bundles at the participating hospitals, as suboptimal compliance and wide variations in the application of sepsis bundles among hospitals have been critical issues in the management of sepsis [1215]. Previous studies [121617] have shown that increased compliance with sepsis bundles improved outcomes and decreased hospital mortality. Considering that sepsis bundle compliance rates increased after the introduction of mandatory reporting via a web-based sepsis registry system in the state of New York [12], we too expect a more accurate identification of compliance rates and a potential increase in sepsis bundle compliance after the implementation of the KISS. This information on sepsis bundle compliance will help improve sepsis management. While the Surviving Sepsis bundle emphasizes the timely administration of broad-spectrum antibiotics to cover possible causative pathogens, this approach may adversely increase the use of broad-spectrum antibiotics and thus the risk of multidrug-resistant pathogens [18]. Therefore, the appropriateness of antimicrobial treatment and antibiotic stewardship in the treatment of sepsis will be assessed. Re-evaluation and de-escalation by antibiotic stewardship are the critical aspects of sepsis management and need to be integrated, along with the sepsis bundle, to improve the quality and outcomes of sepsis management [18]. In Europe (Ireland and United Kingdom), to address this issue, goals and effectiveness of antibiotic stewardship efforts have been made integral parts of sepsis management [1920]. Furthermore, a previous study demonstrated that early antibiotic stewardship interventions significantly increased the appropriateness of antibiotic treatment in patients with sepsis through de-escalation [21]. However, not much is known regarding the impact of sepsis bundles and antibiotic stewardship in sepsis management in the Korea. The findings from the planned case-control studies will therefore be used to estimate the impact of sepsis bundles and antibiotic stewardship on 30-day mortality, multidrug-resistant bacterial pathogen infection rates, consumption of antibiotics, and durations of hospital stay.
Other potential benefits of the KISS are the monthly audits and the data monitoring through a web-based data management system. This is similar to the mandatory sepsis reporting system in the state of New York [12]. Other countries have also adopted such registry systems. For example, all intensive care units in Australia and New Zealand are now contributing to an outcome evaluation registry, the ANZIS CORE registry [22]. The ANZIS CORE registry collects data and reports back to the participating intensive care units after the audit process. In line with these registry systems in other countries, the KISS will contribute to improved quality assurance of data management through the audit and feedback process. The KISS will also enable the participating hospitals to check the data and update them through distinct query and notification features.
The approach described in this study protocol has some limitations. Enrollment of sepsis cases will occur on a monthly basis, and data will be collected from the participating hospitals, which might lead to some selection and sampling biases. In addition, confounding effects from unmeasured variables might affect the analyses. However, the application of multiple logistic regression and propensity scoring will minimize the risks of such biases in our analyses. Moreover, recruitment of additional participating hospitals during the study period and accumulative analyses are expected to minimize such limitations.
In conclusion, the KISS aims to build the comprehensive web-based sepsis registry in the Korea based on a nation-wide network of participating hospitals. The data that will be collected and analyzed will provide information of sepsis epidemiology, application of sepsis bundles, and the impact of antibiotic stewardship efforts in real-life settings. This valuable information can then be used for further improvements in the clinical management of sepsis. Furthermore, the KISS will facilitate research that may lead to the formulation of public health policies regarding the sepsis bundle and antibiotic stewardship strategies in the Korea.
Notes
Conflict of Interest: DGL is editor-in-chief of Journal Infect Chemother; however, he did not involve in the peer reviewer selection, evaluation, and decision process of this article. Otherwise, no potential conflicts of interest relevant to this article was reported.
HBK is an editorial board member of Journal Infect Chemother; however, he did not involve in the peer reviewer selection, evaluation, and decision process of this article. Otherwise, no potential conflicts of interest relevant to this article was reported.
No conflicts of interest for other authors.
References
1. Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K. International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. 2016; 193:259–272. PMID: 26414292.

2. Torio CM, Moore BJ. National inpatient hospital costs: The most expensive conditions by Payer, 2013: Statistical Brief #204. Accessed 8 February 2020. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.jsp?utm_source=AHRQ&utm_medium=AHRQSTAT&utm_content=Content&utm_term=HCUP&utm_campaign=AHRQ_SB_204_2016.
3. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001; 29:1303–1310. PMID: 11445675.

4. Park M, Kim K, Lee JH, Kang C, Jo YH, Kim DH, Kang KW, Lee SH, Park C, Kim J, Chung H, Park H, Jang S. Awareness and knowledge of sepsis in the general Korean population: comparison with the awareness and knowledge of acute myocardial infarction and stroke. Clin Exp Emerg Med. 2014; 1:41–48. PMID: 27752551.

5. Park DW, Chun BC, Kim JM, Sohn JW, Peck KR, Kim YS, Choi YH, Choi JY, Kim SI, Eom JS, Kim HY, Song JY, Song YG, Choi HJ, Kim MJ. Epidemiological and clinical characteristics of community-acquired severe sepsis and septic shock: a prospective observational study in 12 university hospitals in Korea. J Korean Med Sci. 2012; 27:1308–1314. PMID: 23166410.

6. Korean Statistical Information Service. Accessed 8 February 2020. Available at: http://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B34E01&vw_cd=MT_ZTITLE&list_id=D11&seqNo=&lang_mode=ko&language=kor&obj_var_id=&itm_id=&conn_path=MT_ZTITLE.
7. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017; 43:304–377. PMID: 28101605.

8. Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, Lemeshow S, Osborn T, Terry KM, Levy MM. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017; 376:2235–2244. PMID: 28528569.

9. Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, Osborn T, Lemeshow S, Chiche JD, Artigas A, Dellinger RP. Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014; 40:1623–1633. PMID: 25270221.

10. Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC. Surviving Sepsis Campaign. The surviving sepsis campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010; 38:367–374. PMID: 20035219.

11. New York State. Department of health: Sepsis. Accessed 8 February 2020. Available at: https://www.health.ny.gov/diseases/conditions/sepsis/.
12. Levy MM, Gesten FC, Phillips GS, Terry KM, Seymour CW, Prescott HC, Friedrich M, Iwashyna TJ, Osborn T, Lemeshow S. Mortality changes associated with mandated public reporting for sepsis. The results of the New York State initiative. Am J Respir Crit Care Med. 2018; 198:1406–1412. PMID: 30189749.

13. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–810. PMID: 26903338.

14. Shin TG, Hwang SY, Kang GH, Kim WY, Ryoo SM, Kim K, Jo YH, Chung SP, Joo YS, Beom JH, Choi SH, Yoon YH, Kwon WY, Lim TH, Han KS, Choi HS, Suh GJ. Korean Shock Society septic shock registry: a preliminary report. Clin Exp Emerg Med. 2017; 4:146–153. PMID: 29026888.

15. Venkatesh AK, Slesinger T, Whittle J, Osborn T, Aaronson E, Rothenberg C, Tarrant N, Goyal P, Yealy DM, Schuur JD. Preliminary performance on the new CMS sepsis-1 national quality measure: early insights from the emergency quality network (E-QUAL). Ann Emerg Med. 2018; 71:10–15.e1. PMID: 28789803.

16. Liu VX, Morehouse JW, Marelich GP, Soule J, Russell T, Skeath M, Adams C, Escobar GJ, Whippy A. Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med. 2016; 193:1264–1270. PMID: 26695114.

17. Scheer CS, Fuchs C, Kuhn SO, Vollmer M, Rehberg S, Friesecke S, Abel P, Balau V, Bandt C, Meissner K, Hahnenkamp K, Gründling M. Quality improvement initiative for severe sepsis and septic shock reduces 90-day mortality: a 7.5-year observational study. Crit Care Med. 2017; 45:241–252. PMID: 27661863.
18. Fitzpatrick F, Tarrant C, Hamilton V, Kiernan FM, Jenkins D, Krockow EM. Sepsis and antimicrobial stewardship: two sides of the same coin. BMJ Qual Saf. 2019; 28:758–761.

19. Health Service Executive (HSE). National sepsis report, 2017. Accessed 8 February 2020. Available at: https://www.hse.ie/eng/services/publications/clinical-strategy-and-programmes/sepsis-annual-report-2017.pdf.
20. NHS England. Technical guidance for refreshing NHS plans 2018/19 annex A: commissioning for quality and innovation (CQUIN) indicator specification 2017-2019. Accessed 8 February 2020. Available at: https://www.england.nhs.uk/publication/cquin-indicator-specification/.
21. Burston J, Adhikari S, Hayen A, Doolan H, Kelly ML, Fu K, Jensen TO, Konecny P. A role for antimicrobial stewardship in clinical sepsis pathways: a prospective interventional study. Infect Control Hosp Epidemiol. 2017; 38:1032–1038. PMID: 28693625.

22. Australian and New Zealand Intensive Care Society (ANZICS). Center for outcome and resource evaluation. Accessed 8 February 2020. Available at: http:// www.anzics.com.au/Pages/CORE/About-CORE.aspx.
SUPPLEMENTARY MATERIAL
Supplementary Table 1
The sepsis cohort data CRF is shown in Supplementary Table 1.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download