1. Sasazuki T, Juji T, Morishima Y, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Engl J Med. 1998; 339:1177–1185. PMID:
9780337.
2. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006; 354:1813–1826. PMID:
16641398.

3. Barker JN, Krepski TP, DeFor TE, Davies SM, Wagner JE, Weisdorf DJ. Searching for unrelated donor hematopoietic stem cells: availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. 2002; 8:257–260. PMID:
12064362.

4. Ciurea SO, Bayraktar UD. "No donor"? Consider a haploidentical transplant. Blood Rev. 2015; 29:63–70. PMID:
25307958.

5. Powles RL, Morgenstern GR, Kay HE, et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet. 1983; 1:612–615. PMID:
6131300.

6. Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985; 313:765–771. PMID:
3897863.

7. Passweg JR, Baldomero H, Bader P, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017; 52:811–817. PMID:
28287639.

8. Luo Y, Xiao H, Lai X, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014; 124:2735–2743. PMID:
25214441.

9. Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974; 18:295–304. PMID:
4153799.

10. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005; 11:945–956. PMID:
16338616.
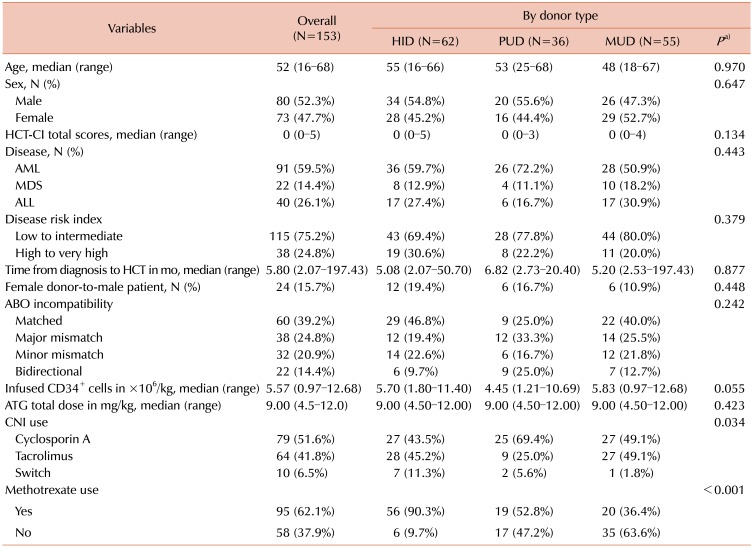
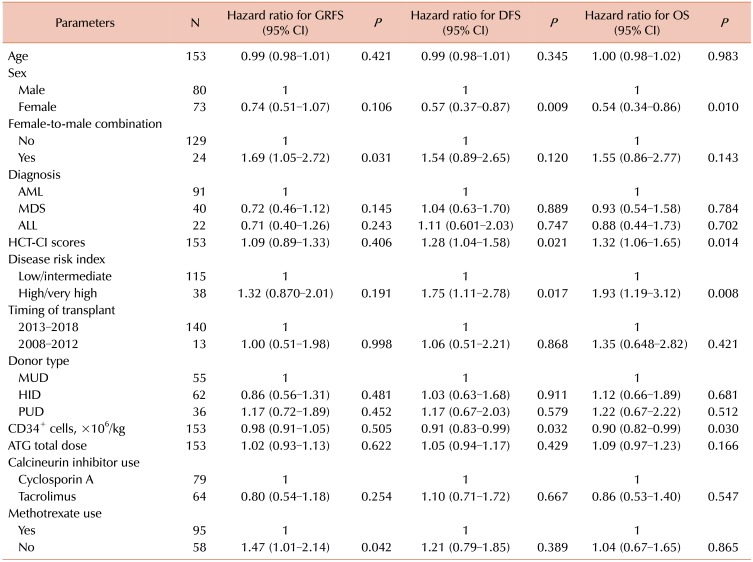
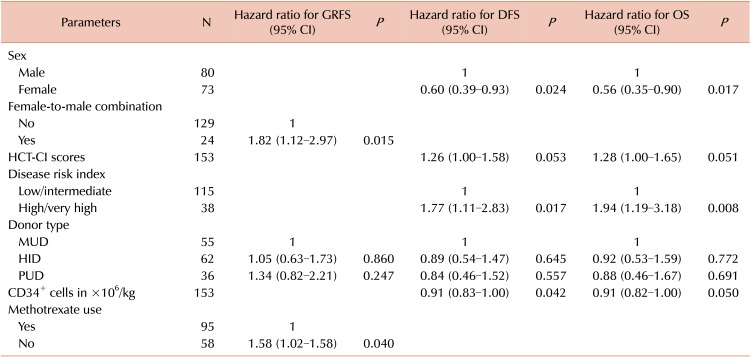
11. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005; 106:2912–2919. PMID:
15994282.

12. Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014; 123:3664–3671. PMID:
24744269.

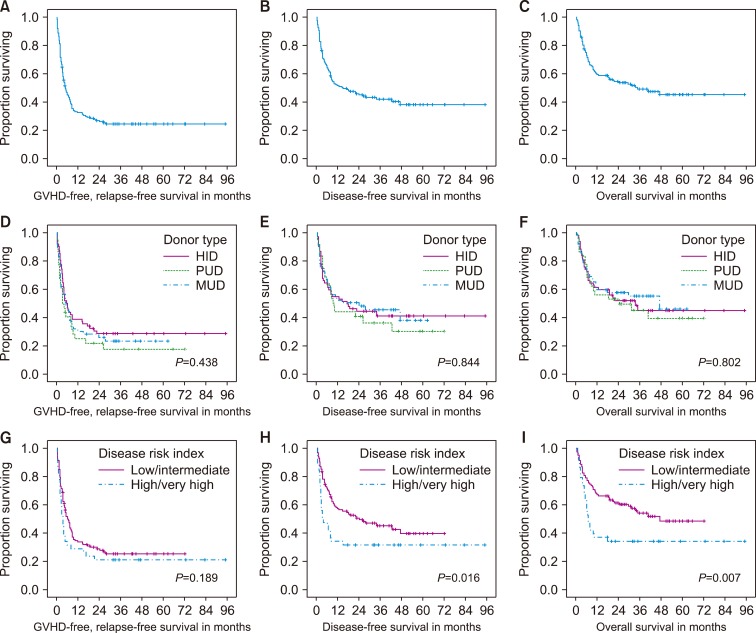
13. Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015; 125:1333–1338. PMID:
25593335.

14. Solh M, Zhang X, Connor K, et al. Factors predicting graftversus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation: multivariable analysis from a single center. Biol Blood Marrow Transplant. 2016; 22:1403–1409. PMID:
27095692.

15. Piemontese S, Ciceri F, Labopin M, et al. A comparison between allogeneic stem cell transplantation from unmanipulated haploidentical and unrelated donors in acute leukemia. J Hematol Oncol. 2017; 10:24. PMID:
28103944.

16. McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015; 125:3024–3031. PMID:
25814532.

17. Kim HT, Zhang MJ, Woolfrey AE, et al. Donor and recipient sex in allogeneic stem cell transplantation: what really matters. Haematologica. 2016; 101:1260–1266. PMID:
27354023.

18. Socié G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011; 117:6375–6382. PMID:
21467544.

19. Kröger N, Solano C, Bonifazi F. Antilymphocyte globulin for chronic graft-versus-host disease. N Engl J Med. 2016; 374:1894–1895.

20. Di Bartolomeo P, Santarone S, De Angelis G, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013; 121:849–857. PMID:
23165479.

21. Wang Y, Liu QF, Xu LP, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015; 125:3956–3962. PMID:
25940714.

22. Lee KH, Lee JH, Lee JH, et al. Reduced-intensity conditioning therapy with busulfan, fludarabine, and antithymocyte globulin for HLA-haploidentical hematopoietic cell transplantation in acute leukemia and myelodysplastic syndrome. Blood. 2011; 118:2609–2617. PMID:
21715313.

23. Lee KH, Lee JH, Lee JH, et al. Reduced-intensity conditioning with busulfan, fludarabine, and antithymocyte globulin for hematopoietic cell transplantation from unrelated or haploidentical family donors in patients with acute myeloid leukemia in remission. Biol Blood Marrow Transplant. 2017; 23:1555–1566. PMID:
28552421.

24. Raiola AM, Dominietto A, di Grazia C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014; 20:1573–1579. PMID:
24910379.

25. Versluis J, Labopin M, Ruggeri A, et al. Alternative donors for allogeneic hematopoietic stem cell transplantation in poor-risk AML in CR1. Blood Adv. 2017; 1:477–485. PMID:
29296964.

26. Chang YJ, Wang Y, Mo XD, et al. Optimal dose of rabbit thymoglobulin in conditioning regimens for unmanipulated, haploidentical, hematopoietic stem cell transplantation: Long-term outcomes of a prospective randomized trial. Cancer. 2017; 123:2881–2892. PMID:
28301690.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download