Abstract
African swine fever (ASF), caused by the ASF virus, a member of the Asfarviridae family, is one of the most important diseases in the swine industry due to its clinical and economic impacts. Since the first report of ASF a century ago, ample information has become available, but prevention and treatment measures are still inadequate. Two waves of epizootic outbreaks have occurred worldwide. While the first wave of the epizootic outbreak was controlled in most of the infected areas, the second wave is currently active in the European and Asian continents, causing severe economic losses to the pig industry. There are different patterns of spreading in the outbreaks between those in European and Asian countries. Prevention and control of ASF are very difficult due to the lack of available vaccines and effective therapeutic measures. However, recent outbreaks in South Korea have been successfully controlled on swine farms, although feral pigs are periodically being found to be positive for the ASF virus. Therefore, we would like to share our story regarding the preparation and application of control measures. The success in controlling ASF on farms in South Korea is largely due to the awareness and education of swine farmers and practitioners, the early detection of infected animals, the implementation of strict control policies by the government, and widespread sharing of information among stakeholders. Based on the experience gained from the outbreaks in South Korea, this review describes the current understanding of the ASF virus and its pathogenic mechanisms, epidemiology, and control.
Go to : 
References
1. Montgomery R. A form of swine fever occurring in British East Africa (Kenya Colony). J Comp Pathol. 1921; 34:159–191.
2. Plowright W, Parker J, Peirce MA. African swine fever virus in ticks (Ornithodoros moubata, murray) collected from animal burrows in Tanzania. Nature. 1969; 221:1071–1073.

3. Sánchez-Cordón PJ, Montoya M, Reis AL, Dixon LK. African swine fever: a re-emerging viral disease threatening the global pig industry. Vet J. 2018; 233:41–48.

4. Arias M, Jurado C, Gallardo C, Fernández-Pinero J, Sánchez-Vizcaíno JM. Gaps in African swine fever: analysis and priorities. Transbound Emerg Dis. 2018; 65(Suppl 1):235–247.

5. Gallardo C, Nieto R, Soler A, Pelayo V, Fernández-Pinero J, Markowska-Daniel I, Pridotkas G, Nurmoja I, Granta R, Simón A, Pérez C, Martín E, Fernández-Pacheco P, Arias M. Assessment of African fever diagnostic techniques as a response to the epidemic outbreak in Eastern European Union countries: how to improve surveillance and control programs. J Clin Microbiol. 2015; 53:2555–2565.

6. Sanchez-Vizcaino JM, Arias M. African swine fever. Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. (eds.).Disease of Swine. 10th ed.pp.p. 396–404. Wiley-Blackwell;Ames: 2012.
7. Bellini S, Rutili D, Guberti V. Preventive measures aimed at minimizing the risk of African swine fever virus spread in pig farming systems. Acta Vet Scand. 2016; 58:82.

8. Gabriel C, Blome S, Malogolovkin A, Parilov S, Kolbasov D, Teifke JP, Beer M. Characterization of African swine fever virus Caucasus isolate in European wild boars. Emerg Infect Dis. 2011; 17:2342–2345.

9. Blome S, Gabriel C, Dietze K, Breithaupt A, Beer M. High virulence of African swine fever virus Caucasus isolate in European wild boars of all ages. Emerg Infect Dis. 2012; 18:708.

10. Penrith ML, Vosloo W. Review of African swine fever: transmission, spread and control. J S Afr Vet Assoc. 2009; 80:58–62.
11. Costard S, Mur L, Lubroth J, Sanchez-Vizcaino JM, Pfeiffer DU. Epidemiology of African swine fever virus. Virus Res. 2013; 173:191–197.

12. Yáñez RJ, Rodríguez JM, Nogal ML, Yuste L, Enríquez C, Rodriguez JF, Viñuela E. Analysis of the complete nucleotide sequence of African swine fever virus. Virology. 1995; 208:249–278.

13. Rodríguez JM, Moreno LT, Alejo A, Lacasta A, Rodríguez F, Salas ML. Genome sequence of African swine fever virus BA71, the virulent parental strain of the nonpathogenic and tissue-culture adapted BA71V. PLoS One. 2015; 10:e0142889.

14. Dixon LK, Chapman DA, Netherton CL, Upton C. African swine fever virus replication and genomics. Virus Res. 2013; 173:3–14.

15. Almendral JM, Almazán F, Blasco R, Viñuela E. Multigene families in African swine fever virus: family 110. J Virol. 1990; 64:2064–2072.

16. González A, Calvo V, Almazán F, Almendral JM, Ramírez JC, de la Vega I, Blasco R, Viñuela E. Multigene families in African swine fever virus: family 360. J Virol. 1990; 64:2073–2081.

17. Vydelingum S, Baylis SA, Bristow C, Smith GL, Dixon LK. Duplicated genes within the variable right end of the genome of a pathogenic isolate of African swine fever virus. J Gen Virol. 1993; 74:2125–2130.

18. Almazán F, Rodríguez JM, Andrés G, Pérez R, Viñuela E, Rodriguez JF. Transcriptional analysis of multigene family 110 of African swine fever virus. J Virol. 1992; 66:6655–6667.

19. Yozawa T, Kutish GF, Afonso CL, Lu Z, Rock DL. Two novel multigene families, 530 and 300, in the terminal variable regions of African swine fever virus genome. Virology. 1994; 202:997–1002.

20. Galindo I, Cuesta-Geijo MA, Hlavova K, Muñoz-Moreno R, Barrado-Gil L, Dominguez J, Alonso C. African swine fever virus infects macrophages, the natural host cells, via clathrin- and cholesterol-dependent endocytosis. Virus Res. 2015; 200:45–55.

21. Hernáez B, Guerra M, Salas ML, Andrés G. African swine fever virus undergoes outer envelope disruption, capsid disassembly and inner envelope fusion before core release from multivesicular endosomes. PLoS Pathog. 2016; 12:e1005595.

22. Sánchez EG, Quintas A, Pérez-Núñez D, Nogal M, Barroso S, CarrascosaÁ L, Revilla Y. African swine fever virus uses macropinocytosis to enter host cells. PLoS Pathog. 2012; 8:e1002754.

23. Sánchez EG, Pérez-Núñez D, Revilla Y. Mechanisms of entry and endosomal pathway of African swine fever virus. Vaccines (Basel). 2017; 5:42.

24. Sánchez-Torres C, Gómez-Puertas P, Gómez-del-Moral M, Alonso F, Escribano JM, Ezquerra A, Domínguez J. Expression of porcine CD163 on monocytes/macrophages correlates with permissiveness to African swine fever infection. Arch Virol. 2003; 148:2307–2323.

25. Popescu L, Gaudreault NN, Whitworth KM, Murgia MV, Nietfeld JC, Mileham A, Samuel M, Wells KD, Prather RS, Rowland RR. Genetically edited pigs lacking CD163 show no resistance following infection with the African swine fever virus isolate, Georgia 2007/1. Virology. 2017; 501:102–106.

26. Carrascosa AL, Sastre I, Viñuela E. African swine fever virus attachment protein. J Virol. 1991; 65:2283–2289.

27. Carrascosa AL, Saastre I, González P, Viñuela E. Localization of the African swine fever virus attachment protein P12 in the virus particle by immunoelectron microscopy. Virology. 1993; 193:460–465.

28. Angulo A, Alcamí A, Viñuela E. Virus-host interactions in African swine fever: the attachment to cellular receptors. Arch Virol Suppl. 1993; 7:169–183.

29. Carrascosa AL, Sastre I, Viñuela E. Production and purification of recombinant African swine fever virus attachment protein p12. J Biotechnol. 1995; 40:73–86.

30. Rojo G, García-Beato R, Viñuela E, Salas ML, Salas J. Replication of African swine fever virus DNA in infected cells. Virology. 1999; 257:524–536.

32. Rodríguez JM, Salas ML, Viñuela E. Intermediate class of mRNAs in African swine fever virus. J Virol. 1996; 70:8584–8589.

33. López-Otín C, Simón-Mateo C, Martínez L, Viñuela E. Gly-Gly-X, a novel consensus sequence for the proteolytic processing of viral and cellular proteins. J Biol Chem. 1989; 264:9107–9110.

34. Simón-Mateo C, Andrés G, Almazán F, Viñuela E. Proteolytic processing in African swine fever virus: evidence for a new structural polyprotein, pp62. J Virol. 1997; 71:5799–5804.

35. Martinez-Pomares L, Simon-Mateo C, Lopez-Otin C, Viñuela E. Characterization of the African swine fever virus structural protein p14.5: a DNA binding protein. Virology. 1997; 229:201–211.

37. Zsak L, Sur JH, Burrage TG, Neilan JG, Rock DL. African swine fever virus (Asfv) multigene families 360 and 530 genes promote infected macrophage survival. Sci World J. 2001; 1:97.

38. Moore DM, Zsak L, Neilan JG, Lu Z, Rock DL. The African swine fever virus thymidine kinase gene is required for efficient replication in swine macrophages and for virulence in swine. J Virol. 1998; 72:10310–10315.

39. Oliveros M, García-Escudero R, Alejo A, Viñuela E, Salas ML, Salas J. African swine fever virus dUTPase is a highly specific enzyme required for efficient replication in swine macrophages. J Virol. 1999; 73:8934–8943.

40. Salguero FJ, Sánchez-Cordón PJ, Sierra MA, Jover A, Núñez A, Gómez-Villamandos JC. Apoptosis of thymocytes in experimental African swine fever virus infection. Histol Histopathol. 2004; 19:77–84.
41. Salguero FJ, Sánchez-Cordón PJ, Núñez A, Fernández de Marco M, Gómez-Villamandos JC. Proinflammatory cytokines induce lymphocyte apoptosis in acute African swine fever infection. J Comp Pathol. 2005; 132:289–302.

42. Hernáez B, Díaz-Gil G, García-Gallo M, Ignacio Quetglas J, Rodríguez-Crespo I, Dixon L, Escribano JM, Alonso C. The African swine fever virus dynein-binding protein p54 induces infected cell apoptosis. FEBS Lett. 2004; 569:224–228.

43. Hurtado C, Granja AG, Bustos MJ, Nogal ML, González de Buitrago G, de Yébenes VG, Salas ML, Revilla Y, Carrascosa AL. The C-type lectin homologue gene (EP153R) of African swine fever virus inhibits apoptosis both in virus infection and in heterologous expression. Virology. 2004; 326:160–170.

44. Banjara S, Caria S, Dixon LK, Hinds MG, Kvansakul M. Structural insight into African swine fever virus A179L-mediated inhibition of apoptosis. J Virol. 2017; 91:e02228–16.

45. Nogal ML, Gonzalez de Buitrago G, Rodriguez C, Cubelos B, Carrascosa AL, Salas ML, Revilla Y. African swine fever virus IAP homologue inhibits caspase activation and promotes cell survival in mammalian cells. J Virol. 2001; 75:2535–2543.

46. Anderson EC, Hutchings GH, Mukarati N, Wilkinson PJ. African swine fever virus infection of the bushpig (Potamochoerus porcus) and its significance in the epidemiology of the disease. Vet Microbiol. 1998; 62:1–15.

47. Oura CA, Powell PP, Anderson E, Parkhouse RM. The pathogenesis of African swine fever in the resistant bushpig. J Gen Virol. 1998; 79:1439–1443.

48. Zsak L, Caler E, Lu Z, Kutish GF, Neilan JG, Rock DL. A nonessential African swine fever virus gene UK is a significant virulence determinant in domestic swine. J Virol. 1998; 72:1028–1035.

49. Sussman MD, Lu Z, Kutish G, Afonso CL, Roberts P, Rock DL. Identification of an African swine fever virus gene with similarity to a myeloid differentiation primary response gene and a neurovirulence-associated gene of herpes simplex virus. J Virol. 1992; 66:5586–5589.

50. Afonso CL, Piccone ME, Zaffuto KM, Neilan J, Kutish GF, Lu Z, Balinsky CA, Gibb TR, Bean TJ, Zsak L, Rock DL. African swine fever virus multigene family 360 and 530 genes affect host interferon response. J Virol. 2004; 78:1858–1864.

51. Kay-Jackson PC, Goatley LC, Cox L, Miskin JE, Parkhouse RM, Wienands J, Dixon LK. The CD2v protein of African swine fever virus interacts with the actin-binding adaptor protein SH3P7. J Gen Virol. 2004; 85:119–130.

52. Borca MV, Kutish GF, Afonso CL, Irusta P, Carrillo C, Brun A, Sussman M, Rock DL. An African swine fever virus gene with similarity to the T-lymphocyte surface antigen CD2 mediates hemadsorption. Virology. 1994; 199:463–468.

53. Borca MV, Carrillo C, Zsak L, Laegreid WW, Kutish GF, Neilan JG, Burrage TG, Rock DL. Deletion of a CD2-like gene, 8-DR, from African swine fever virus affects viral infection in domestic swine. J Virol. 1998; 72:2881–2889.

54. Boinas FS, Hutchings GH, Dixon LK, Wilkinson PJ. Characterization of pathogenic and non-pathogenic African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. J Gen Virol. 2004; 85:2177–2187.

55. Gallardo C, Soler A, Rodze I, Nieto R, Cano-Gómez C, Fernandez-Pinero J, Arias M. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound Emerg Dis. 2019; 66:1399–1404.

56. Burmakina G, Malogolovkin A, Tulman ER, Zsak L, Delhon G, Diel DG, Shobogorov NM, Morgunov YP, Morgunov SY, Kutish GF, Kolbasov D, Rock DL. African swine fever virus serotype-specific proteins are significant protective antigens for African swine fever. J Gen Virol. 2016; 97:1670–1675.

57. Monteagudo PL, Lacasta A, López E, Bosch L, Collado J, Pina-Pedrero S, Correa-Fiz F, Accensi F, Navas MJ, Vidal E, Bustos MJ, Rodríguez JM, Gallei A, Nikolin V, Salas ML, Rodríguez F. BA71 delta CD2: a new recombinant live attenuated African swine fever virus with cross-protective capabilities. J Virol. 2017; 91:e01058–17.
58. Salguero FJ, Ruiz-Villamor E, Bautista MJ, Sánchez-Cordón PJ, Carrasco L, Gómez-Villamandos JC. Changes in macrophages in spleen and lymph nodes during acute African swine fever: expression of cytokines. Vet Immunol Immunopathol. 2002; 90:11–22.

59. Revilla Y, Callejo M, Rodríguez JM, Culebras E, Nogal ML, Salas ML, Viñuela E, Fresno M. Inhibition of nuclear factor κ B activation by a virus-encoded Iκ B-like protein. J Biol Chem. 1998; 273:5405–5411.

60. Neilan JG, Lu Z, Kutish GF, Zsak L, Lewis TL, Rock DL. A conserved African swine fever virus Iκ B homolog, 5EL, is nonessential for growth in vitro and virulence in domestic swine. Virology. 1997; 235:377–385.

61. Gomez-Villamandos JC, Bautista MJ, Sanchez-Cordon PJ, Carrasco L. Pathology of African swine fever: the role of monocyte-mediated protective immune response. Virus Res. 2013; 173:140–149.
62. Sánchez-Vizcaíno JM, Mur L, Gomez-Villamandos JC, Carrasco L. An update on the epidemiology and pathology of African swine fever. J Comp Pathol. 2015; 152:9–21.
63. Gallardo C, Soler A, Nieto R, Sánchez MA, Martins C, Pelayo V, Carrascosa A, Revilla Y, Simón A, Briones V, Sánchez-Vizcaíno JM, Arias M. Experimental transmission of African swine fever (ASF) low virulent isolate NH/P68 by surviving pigs. Transbound Emerg Dis. 2015; 62:612–622.

64. Kalenzi Atuhaire D, Ochwo S, Afayoa M, Norbert Mwiine F, Kokas I, Arinaitwe E, Ademun-Okurut RA, Boniface Okuni J, Nanteza A, Ayebazibwe C, Okedi L, Olaho-Mukani W, Ojok L. Epidemiological overview of African swine fever in Uganda (2001–2012). J Vet Med. 2013; 2013:949638.

65. Thomas LF, Bishop RP, Onzere C, Mcintosh MT, Lemire KA, de Glanville WA, Cook EA, Fèvre EM. Evidence for the presence of African swine fever virus in an endemic region of western Kenya in the absence of any reported outbreak. BMC Vet Res. 2016; 12:192.

66. Wardley RC, de M Andrade C, Black DN, de Castro Portugal FL, Enjuanes L, Hess WR, Mebus C, Ordas A, Rutili D, Sanchez Vizcaino J, Vigario JD, Wilkinson PJ, Moura Nunes JF, Thomson G. African swine fever virus. Brief review. Arch Virol. 1983; 76:73–90.
67. EFSA Panel on Animal Health and Welfare (AHAW). Scientific opinion on African swine fever. EFSA J. 2010; 8:1556.
68. Botija CS. African swine fever. New developments. Rev Sci Tech Off Int Epiz. 1982; 1:1065–1094.
69. Mur L, Boadella M, Martínez-López B, Gallardo C, Gortazar C, Sánchez-Vizcaíno JM. Monitoring of African swine fever in the wild boar population of the most recent endemic area of Spain. Transbound Emerg Dis. 2012; 59:526–531.

70. Beltrán-Alcrudo D, Lubroth J, Depner K, De La Rocque S. African swine fever in the Caucasus. FAO Empres Watch. 2008. 1–8.
71. Gogin A, Gerasimov V, Malogolovkin A, Kolbasov D. African swine fever in the North Caucasus region and the Russian Federation in years 2007–2012. Virus Res. 2013; 173:198–203.

72. Kolbasov D, Titov I, Tsybanov S, Gogin A, Malogolovkin A. African swine fever virus, Siberia, Russia, 2017. Emerg Infect Dis. 2018; 24:796–798.

73. Zhou X, Li N, Luo Y, Liu Y, Miao F, Chen T, Zhang S, Cao P, Li X, Tian K, Qiu HJ, Hu R. Emergence of African swine fever in China. Transbound Emerg Dis. 2018; 65:1482–1484.
74. Feng Y, Zhao T, Nguyen T, Inui K, Ma Y, Nguyen TH, Nguyen VC, Liu D, Bui QA, To LT, Wang C, Tian K, Gao GF. Porcine respiratory and reproductive syndrome virus variants, Vietnam and China, 2007. Emerg Infect Dis. 2008; 14:1774–1776.

75. Vui DT, Tung N, Inui K, Slater S, Nilubol D. Complete genome sequence of porcine epidemic diarrhea virus in Vietnam. Genome Announc. 2014; 2:e00753–14.

76. Wang WH, Lin CY, Chang Ishcol MR, Urbina AN, Assavalapsakul W, Thitithanyanont A, Lu PL, Chen YH, Wang SF. Detection of African swine fever virus in pork products brought to Taiwan by travellers. Emerg Microbes Infect. 2019; 8:1000–1002.

77. Kim HJ, Cho KH, Lee SK, Kim DY, Nah JJ, Kim HJ, Kim HJ, Hwang JY, Sohn HJ, Choi JG, Kang HE, Kim YJ. Outbreak of African swine fever in South Korea, 2019. Transbound Emerg Dis. 2020; 67:473–475.

78. Kim HJ, Lee MJ, Lee SK, Kim DY, Seo SJ, Kang HE, Nam HM. African swine fever virus in pork brought into South Korea by travelers from China, August 2018. Emerg Infect Dis. 2019; 25:1231–1233.

79. Gaudreault NN, Richt JA. Subunit vaccine approaches for African swine fever virus. Vaccines (Basel). 2019; 7:56.

80. Sánchez EG, Pérez-Núñez D, Revilla Y. Development of vaccines against African swine fever virus. Virus Res. 2019; 265:150–155.

81. Teklue T, Sun Y, Abld M, Luo Y, Qiu HJ. Current status and evolving approaches to African swine fever vaccine development. Transbound Emerg Dis. 2020; 67:529–542.

82. Tlaxca JL, Ellis S, Remmele RL Jr. Live attenuated and inactivated viral vaccine formulation and nasal delivery: potential and challenges. Adv Drug Deliv Rev. 2015; 93:56–78.

83. Leitão A, Cartaxeiro C, Coelho R, Cruz B, Parkhouse RM, Portugal FC, Vigário JD, Martins CL. The non-haemadsorbing African swine fever virus isolate ASFV/NH/P68 provides a model for defining the protective anti-virus immune response. J Gen Virol. 2001; 82:513–523.

85. Blome S, Gabriel C, Beer M. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine. 2014; 32:3879–3882.

86. Borca MV, Irusta P, Carrillo C, Afonso CL, Burrage T, Rock DL. African swine fever virus structural protein p72 contains a conformational neutralizing epitope. Virology. 1994; 201:413–418.

87. Ruiz Gonzalvo F, Carnero ME, Caballero C, Martínez J. Inhibition of African swine fever infection in the presence of immune sera in vivo and in vitro. Am J Vet Res. 1986; 47:1249–1252.
88. Zsak L, Onisk DV, Afonso CL, Rock DL. Virulent African swine fever virus isolates are neutralized by swine immune serum and by monoclonal antibodies recognizing a 72-kDa viral protein. Virology. 1993; 196:596–602.

89. Gómez-Puertas P, Oviedo JM, Rodríguez F, Coll J, Escribano JM. Neutralization susceptibility of African swine fever virus is dependent on the phospholipid composition of viral particles. Virology. 1997; 228:180–189.

90. Borca MV, Carrillo C, Zsak L, Laegreid WW, Kutish GF, Neilan JG, Burrage TG, Rock DL. Deletion of a CD2-like gene, 8-DR, from African swine fever virus affects viral infection in domestic swine. J Virol. 1998; 72:2881–2889.

91. Alonso F, Domínguez J, Viñuela E, Revilla Y. African swine fever virus-specific cytotoxic T lymphocytes recognize the 32 kDa immediate early protein (vp32). Virus Res. 1997; 49:123–130.

92. Leitão A, Malur A, Cornelis P, Martins CL. Identification of a 25-aminoacid sequence from the major African swine fever virus structural protein VP72 recognised by porcine cytotoxic T lymphocytes using a lipoprotein based expression system. J Virol Methods. 1998; 75:113–119.

93. Gómez-Puertas P, Rodríguez F, Oviedo JM, Ramiro-Ibáñez F, Ruiz-Gonzalvo F, Alonso C, Escribano JM. Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. J Virol. 1996; 70:5689–5694.

94. Gómez-Puertas P, Rodríguez F, Oviedo JM, Brun A, Alonso C, Escribano JM. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology. 1998; 243:461–471.

95. Barderas MG, Rodríguez F, Gómez-Puertas P, Avilés M, Beitia F, Alonso C, Escribano JM. Antigenic and immunogenic properties of a chimera of two immunodominant African swine fever virus proteins. Arch Virol. 2001; 146:1681–1691.

96. Neilan JG, Zsak L, Lu Z, Burrage TG, Kutish GF, Rock DL. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology. 2004; 319:337–342.

Go to : 
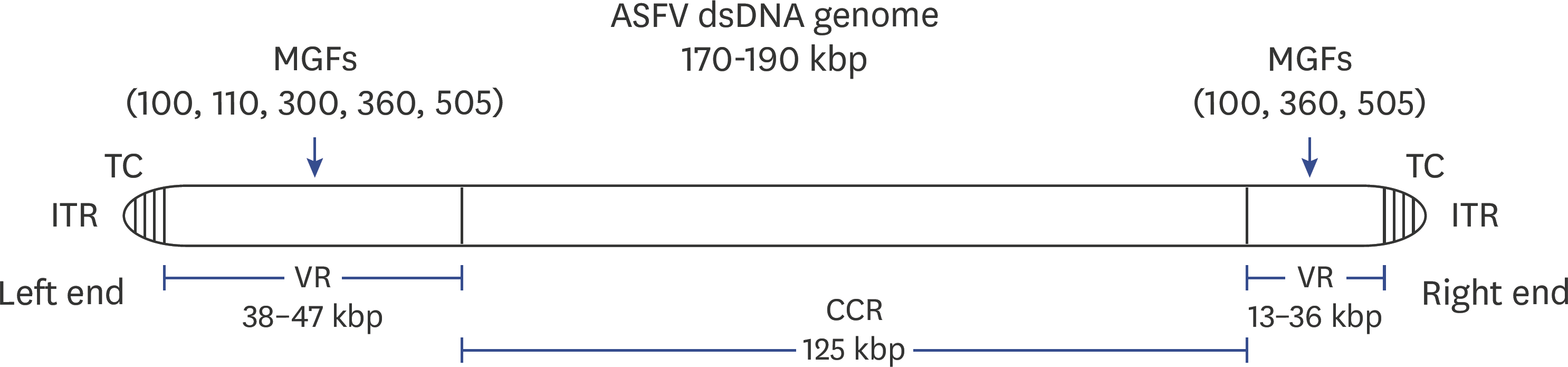 | Fig. 1.Genome structure of ASFV. Numbers in parentheses indicate respective MGFs (e.g., 360 indicates MGF360). ASFV, African swine fever virus; MGF, multigene family; VR, variable region; CCR, central conserved region; ITR, inverted terminal repeat; TC, terminal crosslinks. |
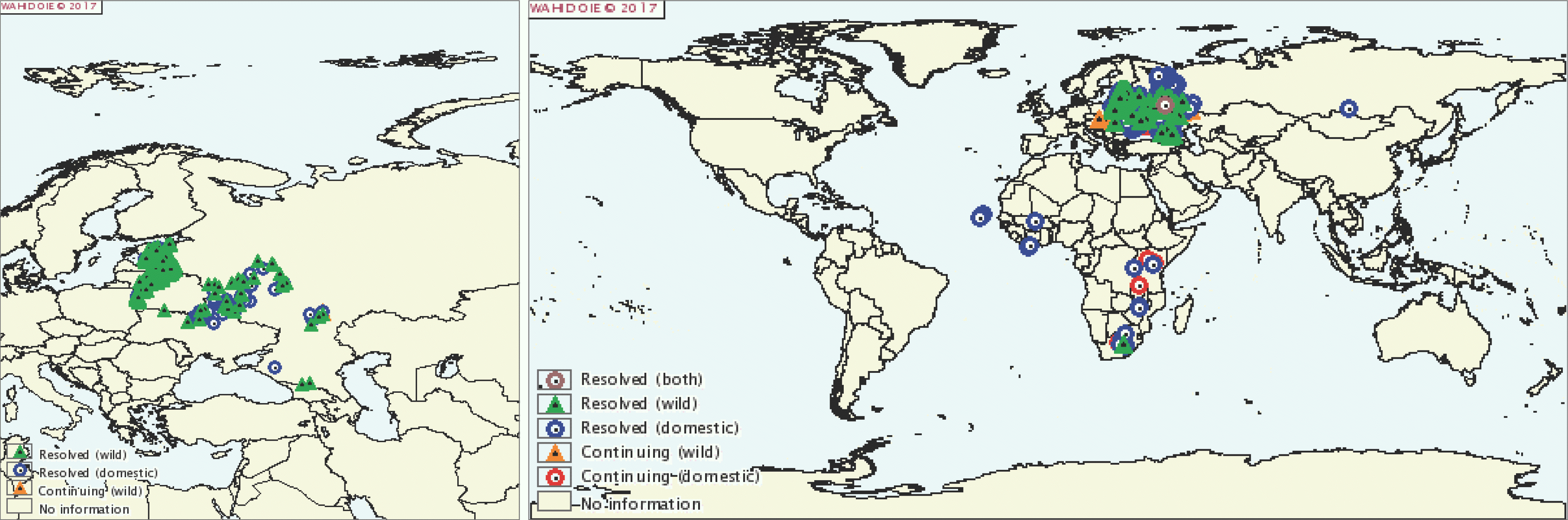 | Fig. 2.On March 18 2017, an ASF breakout occurred in Russia near its border with Mongolia (OIE). ASF, African swine fever; OIE, World Organization of Animal Health. |
 | Fig. 3.Map of ASF outbreaks in South Korea. Fourteen cases of ASF in domestic pigs and 406 cases of ASF in wild boars have been reported near the demilitarized zone between North and South Korea. The yellow, red, and violet colors indicate ASF cases in the first outbreak and in domestic and wild pigs, respectively. ASF, African swine fever. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download