Abstract
Objectives
Galla chinensis inhibited the adherence of planktonic oral bacteria and acid production by cariogenic bacteria. However, little is known about the relevant conditions of Galla Chinensis extract (GCE) exposure time and concentration and the effect of GCE on the structural and functional activity of cariogenic bacteria. The antibacterial effects of natural G. Chinensis extract on S. mutans, S. sanguinis, and S. oralis biofilms were evaluated in vitro.
Methods
Biofilms formed on glass surfaces were treated with different concentrations of GCE at different exposure times. The effects were assessed by examining the bactericidal activity, acido-genesis, minimum inhibitory concentration, and morphology.
Results
There was a statistically significant difference in the bacterial growth inhibition depending on the concentration of the GCE, with bacterial growth being inhibited as the concentration of GCE increased. A concentration of 1.0 mg/ml GCE had similar bactericidal effects against S. mutans and S. oralis biofilms to those produced by 2.0 mg/ml CHX. In the 1.0 mg/ml GCE group, incomplete septa were also observed in the outline of the cell wall, together with disruption of the cell membrane. In addition, there was also a slight exudation of the intracellular content from the bacteria in the 1.0 mg/ml GCE and 2 mg/ml CHX groups.
References
1. Hamada S, Koga T, Ooshima T. Virulence factors of Streptococcus mutans and dental caries prevention. J Dent Res. 1984; 63:407–411.

3. Monchois V, Willemot RM, Monsan P. Glucansucrases: mechanism of action and structure-function relationships. FEMS Microbiol Rev. 1999; 23:131–151.

4. Scheie AA. Modes of action currently known chemical antiplaque agents other than chlorhexidine. J Dent Res. 1989; 68:1609–1616.
5. Zhang TT, Guo HJ, Liu XJ, Chu JP, Zhou XD. Galla chinensis compounds remineralize enamel caries lesions in a rat model. Caries Res. 2016; 50:159–165.

6. Cheynier V. Polyphenols in foods are more complex than often thought. Am J Clin Nutr. 2005; 81:223S–229S.

7. Tagashira M, Uchiyama K, Yoshimura T, Shirota M, Uemitsu N. Ini-hibition by hop bract polyphenols of cellular adherence and water-insoluble glucan synthesis of mutans streptococci. Biosci Biotechnol Biochem. 1997; 61:332–335.
8. Furiga A, Lonvaud-Funel A, Dorignac G, Badet C. In vitro anti-bacterial and anti-adherence effects of natural polyphenolic compunds on oral bacteria. J Appl Microbiol. 2008; 105:1470–1476.
9. Huang S, Gao S, Cheng L, Yu H. Combined effects of nano-hydroxyapatite and Galla Chinensis on remineralization of initial enamel lesion in vitro. J Dent. 2010; 38:811–819.
11. Haffajee AD, Socransky SS. Introduction to microbial aspects of periodontal biofilm communities, development and treatment. Peri-odontol 2000. 2006; 42:7–12.

12. Nascimento GG, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol. 2000; 31:247–256.

13. Xie Q, Li J, Zhou X. Anticaries effect of compounds extracted from Galla Chinensis in a multispecies biofilm model. Oral Microbiol Im-munol. 2008; 23:459–465.
14. National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved Standard M7-A5. NCCLS;Wayne, PA, USA:
15. Clinical and Laboratory Standards Institute. Methods for antimicro-bial susceptibility testing of anaerobic bacteria; approved standard M11-A8. 8th ed.PA, USA: CLSI;2012.
16. Gamboa F, Estupinan M, Galindo A. Presence of streptococcus mutans in saliva and its relationship with dental caries: antimicrobial susceptibility of the isolates. Universitas Scientiarum. 2004; 9:23–27.
17. Huang X, Deng M, Liu M, Cheng L, Exterkate RAM, Li J, et al. Comparison of composition and anticaries effect of Galla Chinensis extracts with different isolation methods. Open Dent J. 2017; 11:447–459.

18. Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001; 48:5–16.

19. Miele WH. Efficacy of grapefruit seed extract against Salmonella typhi, Escherichia coli, and Staphylococcus aureus, Microbiological food analysis report reviewed and approved by Southern Testing and Research Laboratories. NC: Wilson Inc;1988. p. 1–5.
20. Kim JE, Kim HE, Hwang JK, Lee HJ, Kwon HK, Kim BI. Antibacterial characteristics of Curcuma xanthorrhiza extract on Streptococcus mutans biofilm. J Microbiol. 2008; 46:228–232.

21. Duarte S, Gregoire S, Singh AP, Vorsa N, Schaich K, Bowen WH, et al. Inhibitory effects of cranberry polyphenols on formation and ac-idogenicity of Streptococcus mutans biofilms. FEMS Microbiol Lett. 2006; 257:50–56.
22. Haslam E. Natural polypheonols (vegetable tannins) as drugs: possible modes of action. J Nat Prod. 1996; 59:205–215.
23. Burt S. Essential oils: Their antibacterial properties and potential applications in foods - a review. Int J Food Microbiol. 2004; 94:223–253.
Fig. 1.
The bacterial growth curve of S. mutans, S. sanguinis and S. oralis biofilms by GCE concentration. After the biofilms had been exposed to the test solutions for 3, 6, 9, 12, and 24 hours, the number of colonies was counted to determine the CFU. There was a significant difference over time at all concentrations.
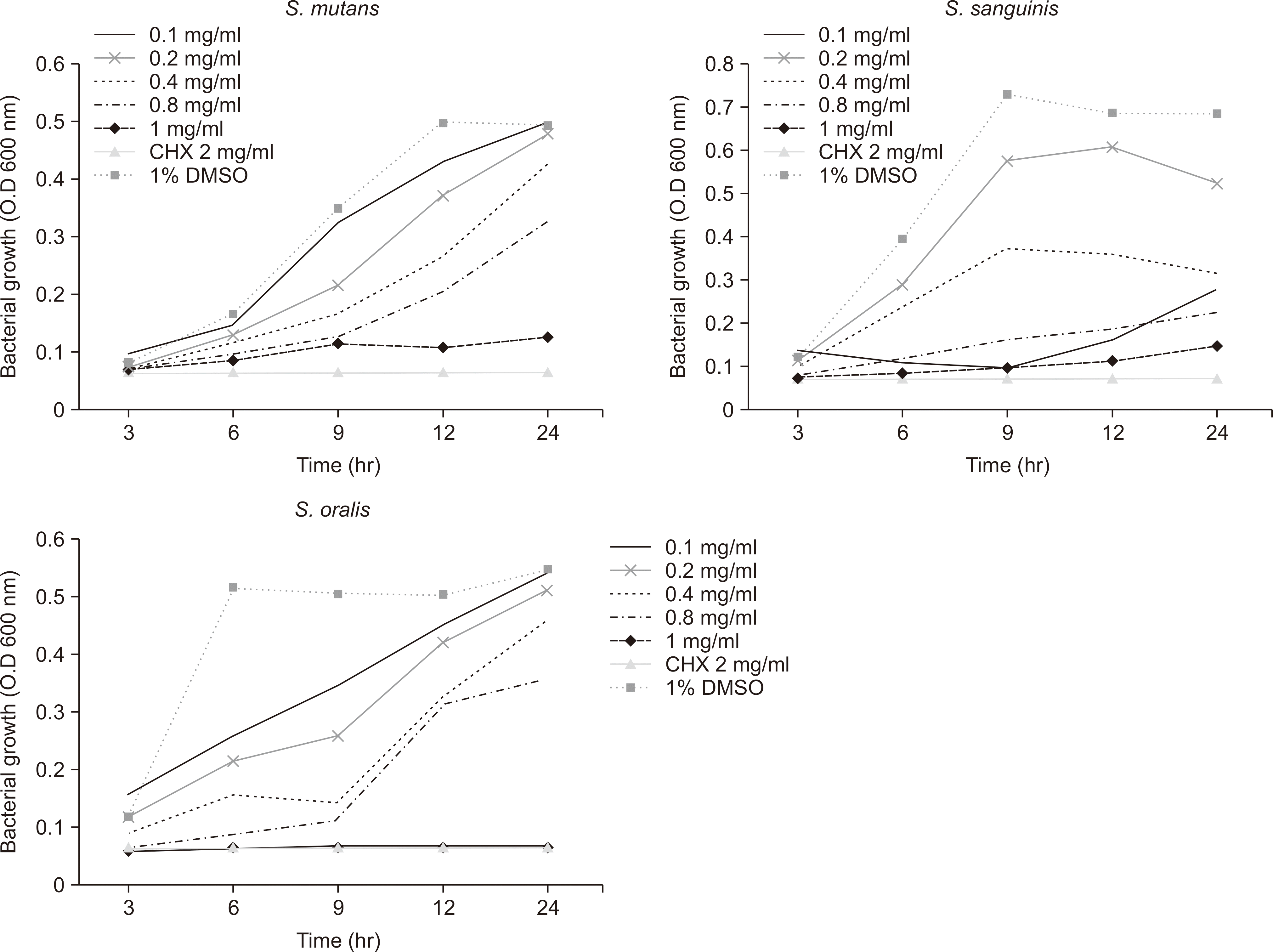
Fig. 2.
TEM images of the S. mutans biofilm after 1 hour of treatment. The black arrows indicate the wall of S. mutans, and the white arrows indicate the intracellular contents. The scale bar is 100 nm. (A) 1% DMSO, (B) 2 mg/ml CHX, and (C) 1.0 mg/ml GCE. After the S. mutans biofilms had been exposed to 1% DMSO for 1 h as the negative control, the TEM showed a clear outline of the S. mutans cell wall and a peptidoglycan layer. However, most of the peptidoglycan layers of S. mutans in the CHX group had disappeared. In the 1.0 mg/ml GCE group, incomplete septa were also observed in the outline of the cell wall. In addition, there was a slight exudation of the intracellular contents in the 1.0 mg/ml GCE and 2 mg/ml CHX groups.
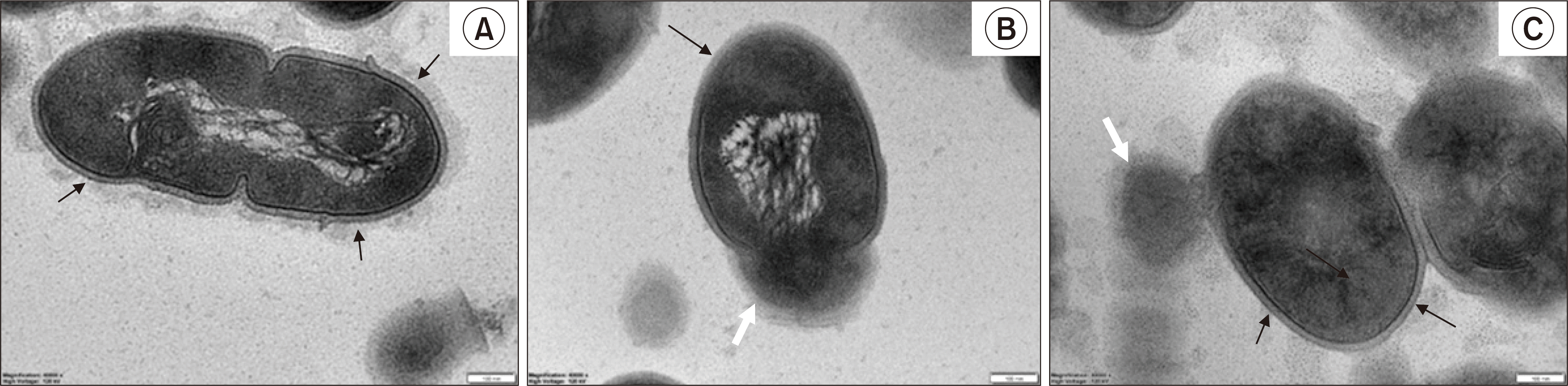
Table 1.
MIC induced by GCE at different concentrations
| Treatment group | S. mutans* | S. sanguis* | S. oralis* | |
|---|---|---|---|---|
| GCE 0.1 mg/ml | M±SD | 2.43±0.04a | 2.58±0.03a | 2.60±0.02a |
| Proportion (%) | 84.04 | 80.51 | 87.18 | |
| GCE 0.2 mg/ml | M±SD | 2.32±0.04a | 2.14±0.12a,b | 2.16±0.04b |
| Proportion (%) | 75.75 | 52.05 | 56.5 | |
| GCE 0.4 mg/ml | M±SD | 1.95±0.14b | 2.10±0.07a,b | 2.41±0.06b |
| Proportion (%) | 47.83 | 50.21 | 48.68 | |
| GCE 0.8 mg/ml | M±SD | 1.69±0.08b | 2.08±0.08a,b | 1.95±0.05c |
| Proportion (%) | 36.96 | 41.51 | 46.18 | |
| GCE 1.0 mg/ml | M±SD | 1.48±0.10c | 1.54±0.12c | 1.35±0.08d |
| Proportion (%) | 30.13 | 28.92 | 25.68 | |
| CHX 2.0 mg/ml | M±SD | 1.40±0.30c | 1.35±0.06c | 1.25±0.05d |
| Proportion (%) | 28.81 | 24.1 | 23.23 | |
| 1% DMSO | M±SD | 2.69±0.05a | 2.69±0.03a,b | 2.74±0.01a |
| Proportion (%) | 102.35 | 89.72 | 107.59 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download