1. Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013; 369:448–457. PMID:
23902484.
2. Libby P. Inflammation in atherosclerosis. Nature. 2002; 420:868–874. PMID:
12490960.
3. Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001; 104:503–516. PMID:
11239408.
4. Allahverdian S, Pannu PS, Francis GA. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc Res. 2012; 95:165–172. PMID:
22345306.
5. Rieg T, Masuda T, Gerasimova M, et al. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol. 2014; 306:F188–93. PMID:
24226519.
6. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012; 8:495–502. PMID:
22310849.
7. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016; 134:752–772. PMID:
27470878.
8. Bhartia M, Tahrani AA, Barnett AH. SGLT-2 inhibitors in development for type 2 diabetes treatment. Rev Diabet Stud. 2011; 8:348–354. PMID:
22262072.
9. Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diabetes Care. 2016; 39:717–725. PMID:
27208375.
10. Henry RR, Rosenstock J, Edelman S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015; 38:412–419. PMID:
25271207.
11. Han JH, Oh TJ, Lee G, et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE
−/− mice fed a western diet. Diabetologia. 2017; 60:364–376. PMID:
27866224.
12. Terasaki M, Hiromura M, Mori Y, et al. Amelioration of hyperglycemia with a sodium-glucose cotransporter 2 inhibitor prevents macrophage-driven atherosclerosis through macrophage foam cell formation suppression in type 1 and type 2 diabetic mice. PLoS One. 2015; 10:e0143396. PMID:
26606676.
13. Leng W, Ouyang X, Lei X, et al. The SGLT-2 inhibitor dapagliflozin has a therapeutic effect on atherosclerosis in diabetic ApoE
−/− mice. Mediators Inflamm. 2016; 2016:6305735. PMID:
28104929.
14. Kim JS, Lee SG, Oh J, et al. Development of advanced atherosclerotic plaque by injection of inflammatory proteins in a rabbit iliac artery model. Yonsei Med J. 2016; 57:1095–1105. PMID:
27401639.
15. Lee SG, Lee SJ, Thuy NV, et al. Synergistic protective effects of a statin and an angiotensin receptor blocker for initiation and progression of atherosclerosis. PLoS One. 2019; 14:e0215604. PMID:
31050669.
16. Tsunoda F, Asztalos IB, Horvath KV, Steiner G, Schaefer EJ, Asztalos BF. Fenofibrate, HDL, and cardiovascular disease in type-2 diabetes: the DAIS trial. Atherosclerosis. 2016; 247:35–39. PMID:
26854974.
17. Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014; 85:962–971. PMID:
24067431.
18. Marx N, McGuire DK. Sodium-glucose cotransporter-2 inhibition for the reduction of cardiovascular events in high-risk patients with diabetes mellitus. Eur Heart J. 2016; 37:3192–3200. PMID:
27153861.
19. Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015; 12:10–17. PMID:
25367649.
20. Peled M, Fisher EA. Dynamic aspects of macrophage polarization during atherosclerosis progression and regression. Front Immunol. 2014; 5:579. PMID:
25429291.
21. Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015; 2015:816460. PMID:
26089604.
22. Pourcet B, Pineda-Torra I. Transcriptional regulation of macrophage arginase 1 expression and its role in atherosclerosis. Trends Cardiovasc Med. 2013; 23:143–152. PMID:
23375628.
23. McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009; 6:410–417. PMID:
19421244.
24. Moriya J. Critical roles of inflammation in atherosclerosis. J Cardiol. 2019; 73:22–27. PMID:
29907363.
25. Xu C, Wang W, Zhong J, et al. Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochem Pharmacol. 2018; 152:45–59. PMID:
29551587.
26. Mancini SJ, Boyd D, Katwan OJ, et al. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci Rep. 2018; 8:5276. PMID:
29588466.
27. Yan ZQ. Regulation of TLR4 expression is a tale about tail. Arterioscler Thromb Vasc Biol. 2006; 26:2582–2584. PMID:
17110607.
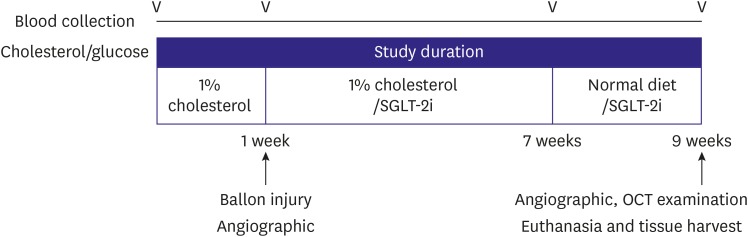
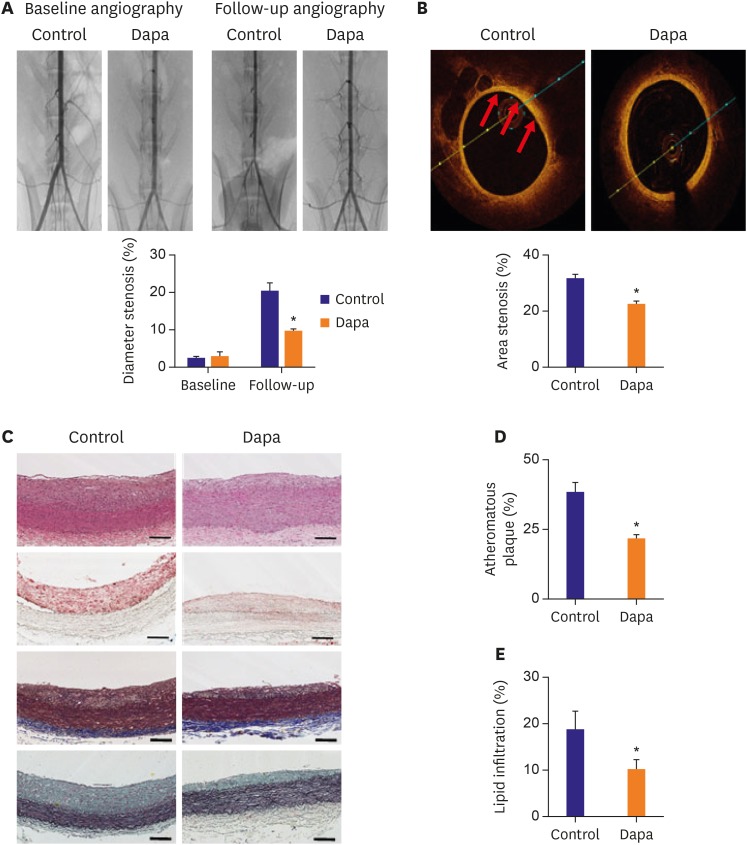
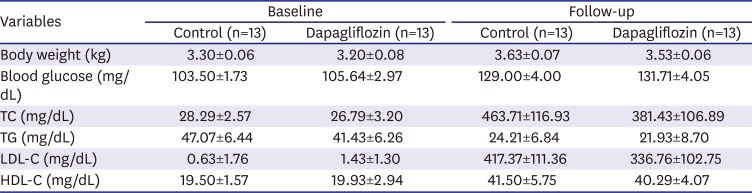
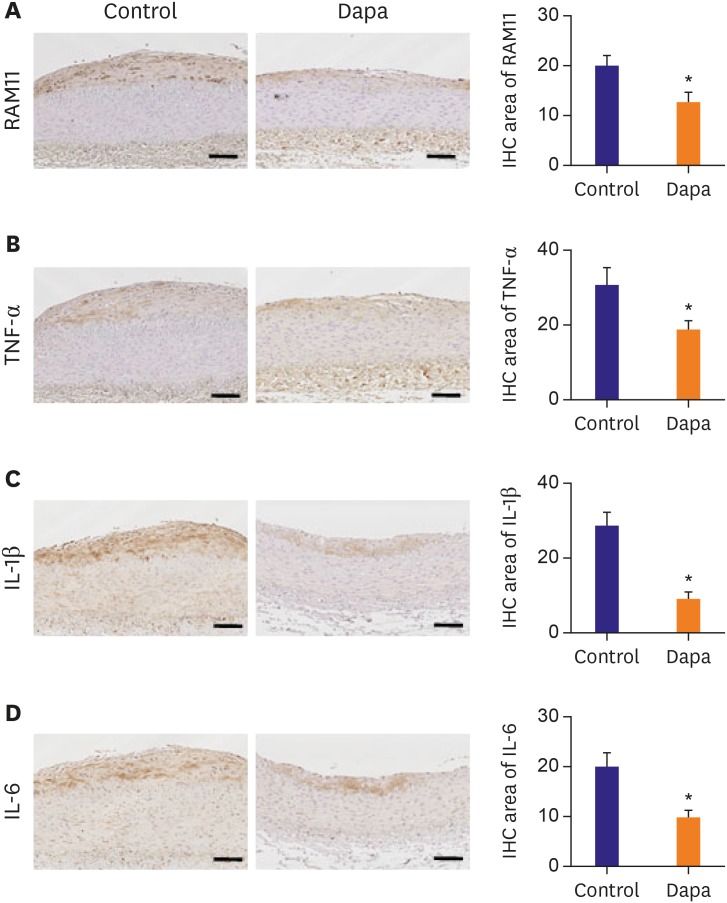




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download