Abstract
Purpose
This study aimed to evaluate the risk of acute myocardial infarction (AMI) associated with intravitreal ranibizumab in age-related macular degeneration (AMD).
Methods
This nationwide retrospective case-crossover study using data from the Korean National Health Insurance Service database included patients diagnosed with exudative AMD using the registration code for exudative AMD (V201) from 2009 to 2014. We identified all incident AMI cases among these exudative AMD cases from inpatient claims and defined the index date as the date of hospitalization. For each patient, we defined the case period as one to 60 days and four control periods as 121 to 180, 181 to 240, 241 to 300, and 301 to 361 days, respectively, before the index date. A prescription of ranibizumab was searched for during the case and control periods. We calculated the adjusted odds ratios and their 95% confidence intervals using a conditional logistic regression model.
Results
From a cohort of patients with exudative AMD (n = 41,860), a total of 181 AMI patients with exudative AMD were included. Among all the patients, 11.05% were treated during the 2 months preceding the index date as compared with 8.29% to 9.39% treated during control periods. The adjusted odds ratio of AMI associated with intravitreal ranibizumab during the preceding 2 months was 1.22 (95% confidence interval, 0.673–2.213; p = 0.5124). Analyses based on case periods of 15 days and 1 month yielded similar results.
Age-related macular degeneration (AMD) is a leading cause of blindness worldwide [1]. Vascular endothelial growth factor (VEGF) was found to play a key role in the development of exudative AMD [23]; hence, VEGF inhibitors have become the mainstay of treatment for this condition [14].
Despite the obvious effectiveness of VEGF inhibitors in restoring and improving the vision of patients with exudative AMD, the nature of possible adverse effects on the systemic vasculature remain uncertain. VEGF has a protective effect against atherothrombotic disease by having important functions in vascular pathophysiology, including the development of collateral circulation, restoration of the damaged endothelium, and hypotensive effects [567]. In contrast, VEGF also stimulates the formation of microvessels inside the atherosclerotic plaque, inducing disease progression [8]. Therefore, anti-VEGF therapies have both inhibitory and promotive effects on atherothrombotic disease, and controversy has arisen as to whether anti-VEGF therapies and their extended administration may lead to an increased risk of systemic thromboembolic events [9].
Some population-based studies have reported that ranibizumab, an anti-VEGF inhibitor developed for AMD treatment, is associated with the development of coronary artery disease [1011]. However, most other studies including pivotal randomized controlled trials (RCTs) have shown no association between coronary artery disease development and ranibizumab treatment [1121314]. Since AMD itself shares similar risk factors and pathologic mechanisms with those of cardiovascular disease [1516], it is necessary to identify whether ranibizumab treatment for AMD further increases the risk of acute myocardial infarction (AMI).
Thus, we conducted a case-crossover study to investigate the association of intravitreal ranibizumab use with the risk of AMI in patients with exudative AMD using nationwide medical claims data in South Korea.
This retrospective nationwide cohort study was approved by the institutional review board of the National Health Insurance Service Ilsan Hospital. The study design was reviewed and approved by the institutional review board of the National Health Insurance Service Ilsan Hospital, Gyeonggi-do, Korea (NHIMC 2015-02-021). The study adhered to the tenets of the Declaration of Helsinki, and the need for written informed consent from participants was waived.
This study used data from the National Health Insurance Service's National Comorbidity Survey (2002–2013) (NHIS-2015-1-069), which was released by the Korean National Health Insurance Service (KNHIS). The database included the entire population of South Korea (n = 47,990,760), and we used the data collected from 2009 to 2014.
All Korean residents are obligated to enroll in the KNHIS and their claims are accompanied by data regarding diagnostic codes, hospitalization, procedures, drug prescriptions, personal information, and information about the hospital. No patient health care records are duplicated because all Korean residents receive a unique identification number at birth. Furthermore, the KNHIS uses the Korean Classification of Diseases (KCDs), which is a system similar to the International Classification of Diseases. In 2007, the NHIS initiated a copayment reduction of up to 90% for patients suffering from 138 rare and intractable disorders, including exudative AMD. Patients with exudative AMD who registered in the program were eligible for the copayment reduction after receiving a confirmed diagnosis from an ophthalmologist based on the NHIS diagnostic criteria. After registration, all exudative AMD-related claims contain the exudative AMD registration code (V201) in addition to the diagnostic code for exudative AMD (H3531).
We identified 41,860 patients with exudative AMD using the registration code for exudative AMD (V201) among beneficiaries 40 years of age or older during the 6-year study period (2009–2014). Among these patients, we identified those who had been hospitalized for AMI (n = 687). The data of patients regarding such were available from 2005, and we established a 4-year washout period by excluding cases identified from 2005 to 2008 to remove any potential preexisting exudative AMD (n = 200). Additionally, patients who were diagnosed with AMI before registration for exudative AMD were excluded (n = 306). Ultimately, we enrolled patients with an incident AMI after their exudative AMD diagnosis as cases (n = 181), defined as those with a primary diagnosis of hospitalization for AMI and coded as KCD I219. The onset of AMI (index date) was defined as the date of the first time of hospitalization with a diagnosis of AMI. Fig. 1 presents the flowchart of subject enrollment. Sociodemographic and clinical characteristics were recorded.
Ranibizumab (0.5 mg only) therapy has been reimbursed by the NHIS since 2009. For this research, we collected drug prescription and intravitreal injection data on the use of ranibizumab. We included patients with both prescription (electronic data interchange [EDI] code 653600320/E01631541) and intravitreal injection (S5070) codes on the same day; thus, there was not a significant time lag between the timing of drug prescription and intravitreal injection. We additionally collected information on patients' age; gender; number of injections; and comorbidities including diabetes mellitus, hypertension, chronic pulmonary disease, chronic liver disease, cardiovascular disease, peptic ulcer disease, dementia, and hemiplegia based on KCD codes (Table 1). Information on the household income and residence of patients was also collected. These factors were selected as candidate risk factors due to their potential association with the risk of AMI. The identified risk factors also served as covariates in assessing the connection between intravitreal ranibizumab injection and the risk of AMI.
We used a case-crossover study design to assess the relationship between intravitreal ranibizumab injections and the risk of AMI in exudative AMD patients. Instead of using matched control subjects, the past experience of a study participant served as their own control. Therefore, stable confounding factors, including those that could not be measured or were unknown, were canceled against each other. The case-crossover design has been widely used as a tool to evaluate drug safety and is particularly suitable when the exposure is intermittent, the effect on risk is immediate and transient, and the outcome is abrupt. It was thought that any potential anti-VEGF antibody-related cardiovascular toxicity would be immediate and transient and the outcome (AMI) would be abrupt such that the case-crossover design was an appropriate method in this study.
For each patient, we defined the case period as one to 60 days and four control periods as 121 to 180, 181 to 240, 241 to 300, and 301 to 361 days, respectively, before the index date. A pharmacy prescription and intravitreal injection database was reviewed for information on ranibizumab use during the case and control periods. Patients were considered to have been treated with intravitreal ranibizumab injection when they had drug prescription and intravitreal injection EDI codes recorded for the same day. We compared intravitreal ranibizumab injection between the case and control periods, calculating the crude and adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) using a conditional logistic regression model. A significance level of 0.05 was considered to be statistically significant. The SAS ver. 9.4 (SAS Institute, Cary, NC, USA) and Stata/SE ver. 12.1 (Stata Corp., College Station, TX, USA) were used to perform the analyses in this study.
A total of 181 patients with AMI and exudative AMD were included. Table 1 lists the demographic features of the study cohort. Less than half (46.41%) of the patients experienced the first AMI in their 70s, and 71% of the patients were male. Patients presented with various systemic comorbidities, including hypertension (4.95%), chronic pulmonary disease (4.40%), and diabetes (4.40%). When the household income of the patients was analyzed, the majority of patients were in the group of over the 70th percentile. In total, 38.12% of the patients lived in a small city, while the rest of the patients' residences were distributed in the order of large city (31.49%), metropolitan (16.57%), and rural areas (13.81%).
Of the 181 patients, 123 had received at least one injection, 120 (66.30%) had received one to three injections, and three (1.66%) had received more than four injections within the 1 year before the incident AMI. Conversely, a total of 58 (32.04%) patients had no injection history within this period (Table 2). Among all the patients, 11.05% had been treated during the preceding 2 months as compared with 8.29% to 9.39% treated during the control periods (Table 3).
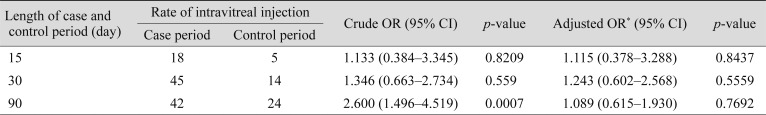
In the multivariate logistic regression analyses, the adjusted OR of AMI associated with intravitreal ranibizumab during the preceding 2 months was 1.220 (95% CI, 0.673–2.213; p = 0.51) (Table 4). In the subgroup analysis for sex and age, intravitreal ranibizumab injection was not significantly associated with an elevated risk of AMI (Table 5). We conducted analyses using different time windows (1–15 and 1–30 days for the case periods, respectively) and did not find any increase in the risk of AMI associated with intravitreal ranibizumab either (Table 6).
We performed a case-crossover analysis using national health insurance claims data and found no increased risk of hospitalization for AMI within 60 days of intravitreal ranibizumab injection in AMD patients when compared with in earlier control periods. Subgroup analyses based on patients' age, sex, and different case periods showed similar results.
The RCT studies that demonstrated the therapeutic efficacy of ranibizumab also analyzed safety-associated thromboembolic events including AMI, but it was difficult to confirm whether the risk of AMI increases due to its rare occurrence and our relatively small sample size [1412131417181920]. In the Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of nAMD (MARINA) [1] and Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration Study (ANCHOR) trials [412], the incidence of AMI was not significantly different between the ranibizumab treatment and sham groups. During the 24-month treatment period, the occurrences of AMI were as follows: four (1.7%, sham), eight (3.4%, 0.3 mg of ranibizumab), and three (1.3%, 0.5 mg of ranibizumab) cases in MARINA and two (1.4%, verteporfin photodynamic therapy [PDT]), one (0.7%, 0.3 mg of ranibizumab), and five (3.6%, 0.5 mg of ranibizumab) cases in ANCHOR. In a phase IIIb, multicenter, randomized, double-masked, sham injection–controlled study of the efficacy and safety of ranibizumab in subjects with subfoveal CNV with or without classic CNV secondary to AMD [13], AMI occurred in only one patient in the sham group and not at all in either the ranibizumab 0.3 mg group or 0.5 mg group. In the RhuFab V2 Ocular Treatment Combining the Use of Visudyne to Evaluate Safety study, which compared the difference between PDT and ranibizumab combined with PDT [14], all three observed events of AMI occurred in the PDT-alone group (5.4%). Among other RCTs studying the effect of various doses of ranibizumab [171819] and treatment schedules [181920], there was no increase of AMI risk due to ranibizumab treatment.
Several meta-analyses using RCTs [2122] and studies with large-size health insurance claims datasets [10112324] have been conducted as part of an effort to clarify the risk of AMI after ranibizumab treatment. Consistent with our results, most of these studies did not show significant increases in AMI risk after intravitreal ranibizumab either. A recent meta-analysis involving 11 trials with AMD reported no apparent influence of ranibizumab in the comparisons of 0.5 versus 0.0 mg, 0.5 versus 0.3 mg, monthly versus pro re nata, 0.3 versus 0.0 mg, and combined regimens [21]. In the previous meta-analysis performed by the same researchers [22], intravitreal ranibizumab was associated with the incidence of cerebrovascular accident (OR, 3.24; 95% CI, 0.96–10.95; p = 0.045), whereas there was no apparent association between intravitreal ranibizumab and AMI (p = 0.193). In the analysis of the risk of adverse events between treatment groups receiving PDT, pegaptanib, bevacizumab, or ranibizumab using claims data, the hazard of myocardial infarction was significantly lower with ranibizumab use than with PDT (hazard ratio, 0.73; 95% CI, 0.58–0.92) [23]. Moreover, a self-controlled case-series analysis, which has similar study design to that of our study, suggested no evidence of an increased risk of AMI with ranibizumab in any of the risk periods (1–30 days incidence rate ratio, 0.90; 95% CI, 0.65–1.23 vs. 31–60 days incidence rate ratio, 0.98; 95% CI, 0.54–1.79) [24].
Conversely, some previous studies have suggested a possible relationship exists between AMI and ranibizumab use. In a whole-population study in Australia [10], although all of the adverse events examined were rare in nature, the risk of 12-month AMI in patients treated with VEGF inhibitors including ranibizumab and bevacizumab was approximately 2.3 to 2.5 times that in a community group in the unadjusted model (95% CI, 1.3–4.9) and following adjustment for prior AMI, diabetes, sex, and age (95% CI, 1.2–4.5). Further, the AMI rate in patients treated with ranibizumab only was greater than that in the community group, although the difference was not statistically significant. In a population-based self-matched crossover analysis involving patients receiving intravitreal ranibizumab and bevacizumab for AMD or macular edema in Canada [11], the rate per 1,000 patients annually for myocardial infarction increased from 3.1 during the baseline interval to 5.1 during the subsequent interval (relative risk, 1.65; 95% CI, 1.43–1.91; p < 0.0001).
To the best of our knowledge, this study is the first to apply a case-crossover design in the investigation of AMD patients to elucidate the association between intravitreal ranibizumab injection and the risk of AMI in Korea. The strength of our study is that it effectively controlled for confounding factors such as smoking, body mass index, cholesterol level, and other comorbidities (e.g., hypertension, diabetes, carotid artery disease) with the case-crossover study design, which used each patient as a control for him- or herself. Additionally, because the number of incident AMI cases among AMD patients treated with ranibizumab is relatively rare, it is difficult to determine the association between ranibizumab therapy and the risk of AMI. However, our study had the capability to evaluate this association using a nationwide large-size claims dataset.
There are some limitations in our study. First, we could not control for the off-label use of intravitreal bevacizumab injection because it is not covered by the national health insurance system. Second, although we used a self-controlled study design to reduce the effect of the confounding factors, the change in systemic medications or improved cardiovascular risk factors during the study period might have affected the incidence of AMI. In this study, hospitalizations for hypertension, diabetes mellitus, and dementia were considered covariates during the regression analysis, but the change in systemic medications could not be controlled for. Third, because we used a large-scale claims dataset from the national insurance system, the sensitivities of the diagnostic codes in determining the risk factors of AMI were not validated by a direct review of the medical records. However, previous studies have reported that the accuracy of the diagnoses in the Korean Health Insurance Database is quite reliable [2526]. Fourth, the influence of comorbidities not reported during the study period could be missed. However, most of the comorbidities are chronic diseases and are thought to have been treated in some fashion or another during the 6 years of the study period. Lastly, if the patient died from AMI without a diagnostic code of AMI, they may be missing from our analysis and the relatively small number of AMD patients who developed AMI may have led to insufficient power in examining the association between AMI and Retinal vascular occlusion.
In conclusion, this case-crossover analysis demonstrated no increased risk of hospitalization for AMI within 60 days of intravitreal ranibizumab treatment in AMD patients. Ranibizumab is an effective treatment for patients with AMD, is well-tolerated, and is not associated with a clinically significant higher risk of AMI.
Acknowledgements
This work was supported by a National Health Insurance Ilsan Hospital grant (NHIMC 2015-02-015). This study used data from Korean National Health Insurance Service (KNHIS) database. The authors alone are responsible for the content and writing of this article.
Notes
References
1. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431. PMID: 17021318.

2. Frank RN, Amin RH, Eliott D, et al. Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol. 1996; 122:393–403. PMID: 8794712.

3. Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996; 37:1929–1934. PMID: 8759365.

4. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444. PMID: 17021319.

5. Simons M. Angiogenesis: where do we stand now? Circulation. 2005; 111:1556–1566. PMID: 15795364.
6. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997; 18:4–25. PMID: 9034784.

7. Henry TD, Annex BH, McKendall GR, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003; 107:1359–1365. PMID: 12642354.
8. Juan-Babot JO, Martinez-Gonzalez J, Berrozpe M, Badimon L. Neovascularization in human coronary arteries with lesions of different severity. Rev Esp Cardiol. 2003; 56:978–986. PMID: 14563292.
9. Tunon J, Ruiz-Moreno JM, Martin-Ventura JL, et al. Cardiovascular risk and antiangiogenic therapy for age-related macular degeneration. Surv Ophthalmol. 2009; 54:339–348. PMID: 19422962.
10. Kemp A, Preen DB, Morlet N, et al. Myocardial infarction after intravitreal vascular endothelial growth factor inhibitors: a whole population study. Retina. 2013; 33:920–927. PMID: 23492942.
11. Schlenker MB, Thiruchelvam D, Redelmeier DA. Intravitreal anti-vascular endothelial growth factor treatment and the risk of thromboembolism. Am J Ophthalmol. 2015; 160:569–580. PMID: 26116264.

12. Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009; 116:57–65. PMID: 19118696.

13. Abraham P, Yue H, Wilson L. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. Am J Ophthalmol. 2010; 150:315–324. PMID: 20598667.

14. Antoszyk AN, Tuomi L, Chung CY, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol. 2008; 145:862–874. PMID: 18321465.

15. Wong TY, Tikellis G, Sun C, et al. Age-related macular degeneration and risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Ophthalmology. 2007; 114:86–91. PMID: 17198851.
16. Wong TY. Age-related macular degeneration and cardiovascular disease in the era of anti-vascular endothelial growth factor therapies. Am J Ophthalmol. 2009; 148:327–329. PMID: 19703607.

17. Boyer DS, Heier JS, Brown DM, et al. A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009; 116:1731–1739. PMID: 19643495.

18. Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013; 120:1046–1056. PMID: 23352196.

19. Schmidt-Erfurth U, Eldem B, Guymer R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology. 2011; 118:831–839. PMID: 21146229.

20. IVAN Study Investigators. Chakravarthy U, Harding SP, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012; 119:1399–1411. PMID: 22578446.
21. Ueta T, Noda Y, Toyama T, et al. Systemic vascular safety of ranibizumab for age-related macular degeneration: systematic review and meta-analysis of randomized trials. Ophthalmology. 2014; 121:2193–2203. PMID: 25023760.
22. Ueta T, Yanagi Y, Tamaki Y, Yamaguchi T. Cerebrovascular accidents in ranibizumab. Ophthalmology. 2009; 116:362.

23. Curtis LH, Hammill BG, Schulman KA, Cousins SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010; 128:1273–1279. PMID: 20937996.

24. Pratt NL, Ramsay EN, Kemp A, et al. Ranibizumab and risk of hospitalisation for ischaemic stroke and myocardial infarction in patients with age-related macular degeneration: a self-controlled case-series analysis. Drug Saf. 2014; 37:1021–1027. PMID: 25260802.

25. Kimm H, Yun JE, Lee SH, et al. Validity of the diagnosis of acute myocardial infarction in korean national medical health insurance claims data: the korean heart study (1). Korean Circ J. 2012; 42:10–15. PMID: 22363378.

26. Park JK, Kim KS, Kim CB, et al. The accuracy of ICD codes for cerebrovascular diseases in medical insurance claims. Korean J Prev Med. 2000; 33:76–82.
Fig. 1
Flowchart of the study cohort. AMD = age-related macular degeneration; AMI = acute myocardial infarction.
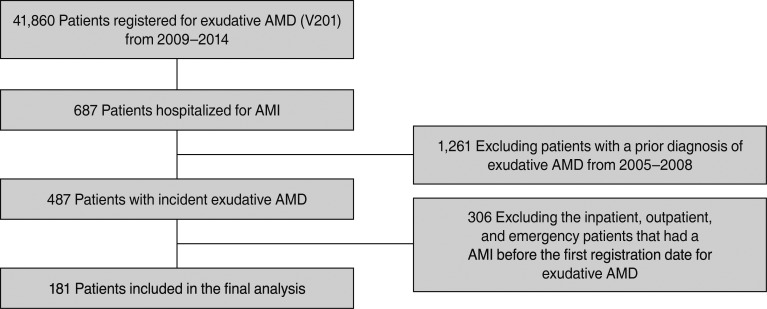
Table 2
Baseline characteristics of the study cohort patients with incident acute myocardial infarction according to exposure to intravitreal ranibizumab injections
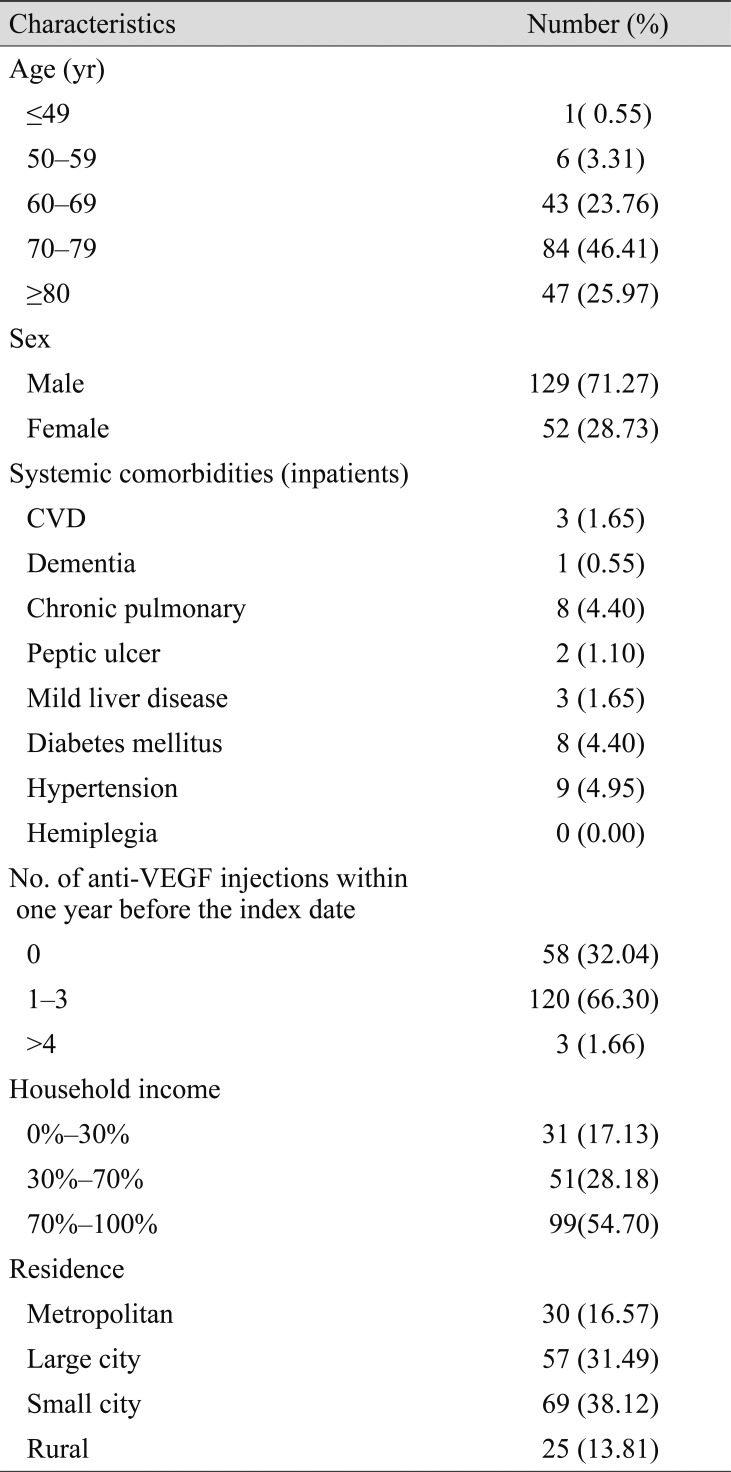
Table 3
Number of patients who underwent intravitreal ranibizumab injection during the periods before incident hospitalization for acute myocardial infarction
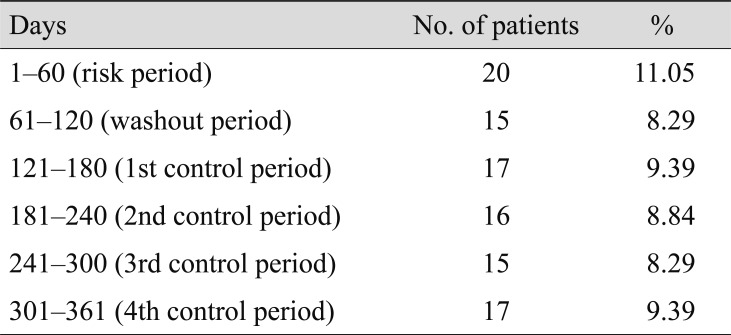
Table 4
Risk of acute myocardial infarction associated with intravitreal ranibizumab injection in the 2 months preceding an incident acute myocardial infarction





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download