1. Katai H, Sano T, Fukagawa T, Shinohara H, Sasako M. Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg. 2003; 90:850–853. PMID:
12854112.

2. Liedman B. Symptoms after total gastrectomy on food intake, body composition, bone metabolism, and quality of life in gastric cancer patients--is reconstruction with a reservoir worthwhile? Nutrition. 1999; 15:677–682. PMID:
10467612.

3. Hosoda K, Yamashita K, Katada N, Moriya H, Mieno H, Shibata T, et al. Potential benefits of laparoscopy-assisted proximal gastrectomy with esophagogastrostomy for cT1 upper-third gastric cancer. Surg Endosc. 2016; 30:3426–3436. PMID:
26511124.

4. Adachi Y, Inoue T, Hagino Y, Shiraishi N, Shimoda K, Kitano S. Surgical results of proximal gastrectomy for early-stage gastric cancer: jejunal interposition and gastric tube reconstruction. Gastric Cancer. 1999; 2:40–45. PMID:
11957069.

5. Muraoka A, Kobayashi M, Kokudo Y. Laparoscopy-assisted proximal gastrectomy with the hinged double flap method. World J Surg. 2016; 40:2419–2424. PMID:
27094564.

6. Huh YJ, Lee HJ, Oh SY, Lee KG, Yang JY, Ahn HS, et al. Clinical outcome of modified laparoscopy-assisted proximal gastrectomy compared to conventional proximal gastrectomy or total gastrectomy for upper-third early gastric cancer with special references to post-operative reflux esophagitis. J Gastric Cancer. 2015; 15:191–200. PMID:
26468417.

7. Hsu CP, Chen CY, Hsieh YH, Hsia JY, Shai SE, Kao CH. Esophageal reflux after total or proximal gastrectomy in patients with adenocarcinoma of the gastric cardia. Am J Gastroenterol. 1997; 92:1347–1350. PMID:
9260804.
8. Ahn SH, Jung DH, Son SY, Lee CM, Park DJ, Kim HH. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. . Gastric Cancer. 2014; 17:562–570. PMID:
24052482.

9. Han WH, Kim YW, Kim DH, Shahin MA, Yun YI. Video of totally laparoscopic modified esophagogastrostomy using a spade shaped anastomosis following proximal gastrectomy (SPADE Operation). J Minim Invasive Surg. 2017; 20:163–165.

10. Delattre JF, Avisse C, Marcus C, Flament JB. Functional anatomy of the gastroesophageal junction. Surg Clin North Am. 2000; 80:241–260. PMID:
10685151.

11. Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996; 111:85–92. PMID:
8698230.

12. Kubo M, Sasako M, Gotoda T, Ono H, Fujishiro M, Saito D, et al. Endoscopic evaluation of the remnant stomach after gastrectomy: proposal for a new classification. Gastric Cancer. 2002; 5:83–89. PMID:
12111583.

13. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017; 20:1–19.
14. Kayani B, Garas G, Arshad M, Athanasiou T, Darzi A, Zacharakis E. Is hand-sewn anastomosis superior to stapled anastomosis following oesophagectomy? Int J Surg. 2014; 12:7–15. PMID:
24239928.

15. Saluja SS, Ray S, Pal S, Sanyal S, Agrawal N, Dash NR, et al. Randomized trial comparing side-to-side stapled and hand-sewn esophagogastric anastomosis in neck. J Gastrointest Surg. 2012; 16:1287–1295. PMID:
22528571.

16. Ohyama S, Tokunaga M, Hiki N, Fukunaga T, Fujisaki J, Seto Y, et al. A clinicopathological study of gastric stump carcinoma following proximal gastrectomy. Gastric Cancer. 2009; 12:88–94. PMID:
19562462.

17. Takeshita K, Saito N, Saeki I, Honda T, Tani M, Kando F, et al. Proximal gastrectomy and jejunal pouch interposition for the treatment of early cancer in the upper third of the stomach: surgical techniques and evaluation of postoperative function. Surgery. 1997; 121:278–286. PMID:
9092128.

18. Kamikawa Y, Kobayashi T, Ueyama S, Satomoto K. A new antireflux procedure in esophagogastrostomy after proximal gastrectomy. Gastroenterol Surg. 2001; 24:1053–1060.
19. Nishigori T, Okabe H, Tsunoda S, Shinohara H, Obama K, Hosogi H, et al. Superiority of laparoscopic proximal gastrectomy with hand-sewn esophagogastrostomy over total gastrectomy in improving postoperative body weight loss and quality of life. Surg Endosc. 2017; 31:3664–3672. PMID:
28078458.

20. Hayami M, Hiki N, Nunobe S, Mine S, Ohashi M, Kumagai K, et al. Clinical outcomes and evaluation of laparoscopic proximal gastrectomy with double-flap technique for early gastric cancer in the upper third of the stomach. Ann Surg Oncol. 2017; 24:1635–1642. PMID:
28130623.

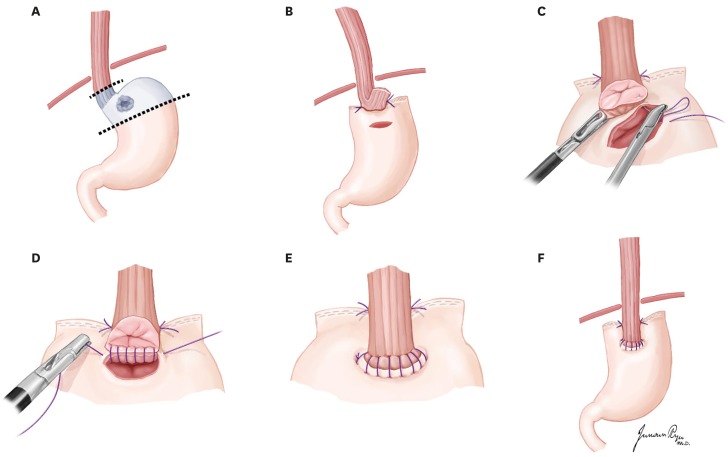
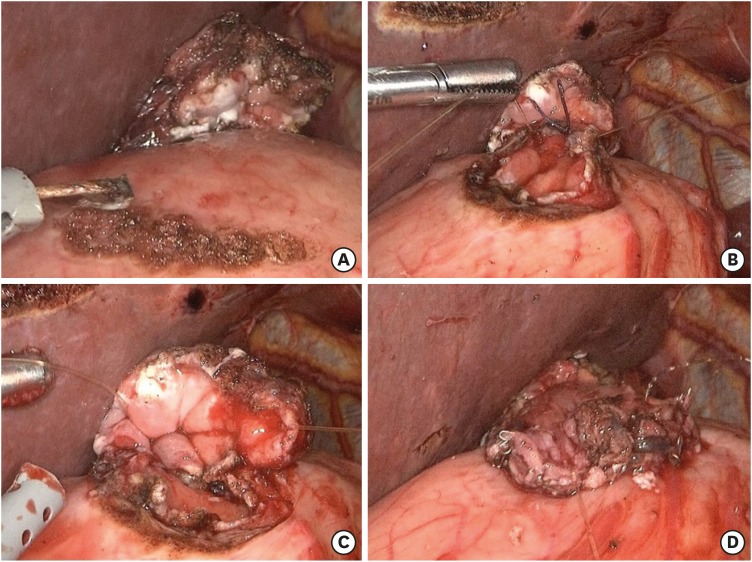
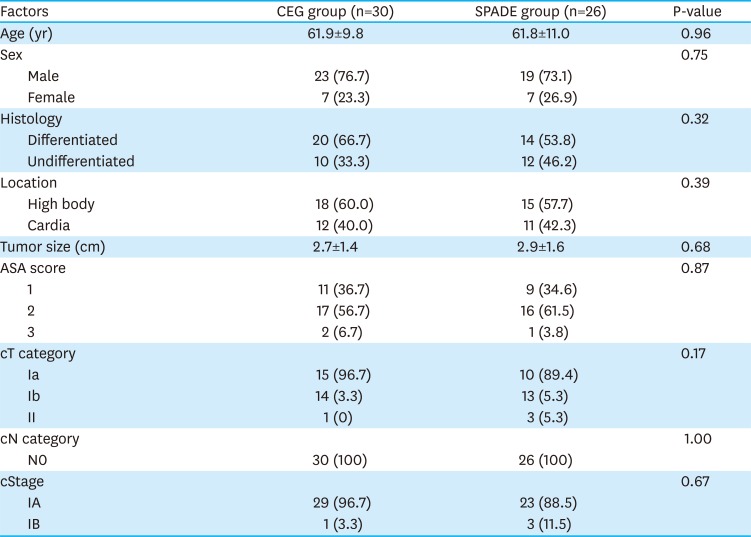
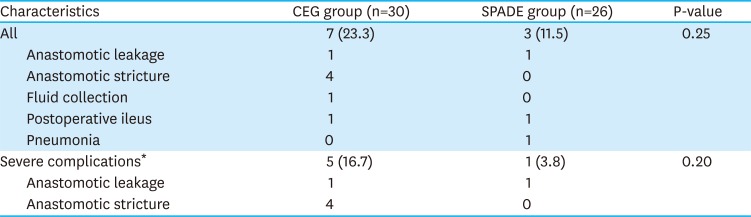





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download