INTRODUCTION
MATERIALS AND METHODS
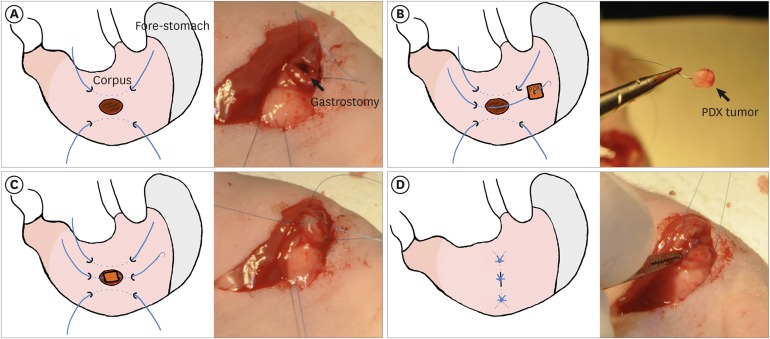
Orthotopic and heterotopic PDX model
Table 1
Clinicopathological characteristics of the donor patients
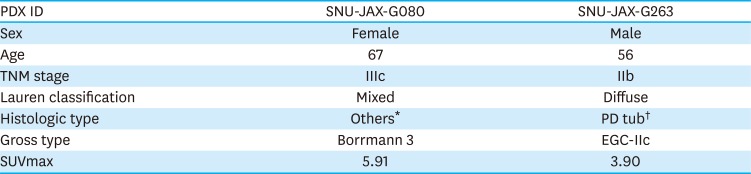
| PDX ID | SNU-JAX-G080 | SNU-JAX-G263 |
|---|---|---|
| Sex | Female | Male |
| Age | 67 | 56 |
| TNM stage | IIIc | IIb |
| Lauren classification | Mixed | Diffuse |
| Histologic type | Others* | PD tub† |
| Gross type | Borrmann 3 | EGC-IIc |
| SUVmax | 5.91 | 3.90 |
Sham-orthotopic model
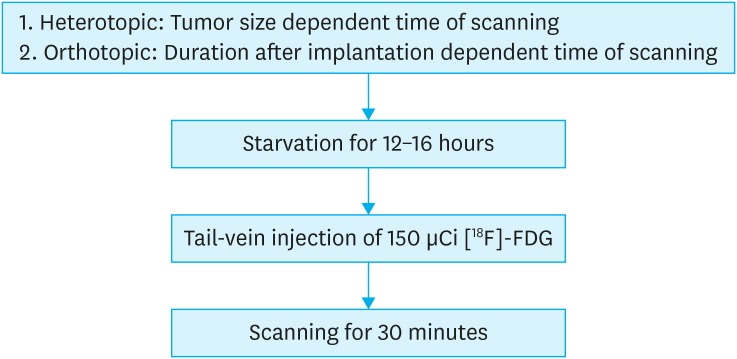
[18F]-FDG-PET/MRI imaging
• Simultaneous PET/MRI scans were acquired for 30 minutes. MRI imaging protocol consisted of T2-weighted fast spin echo sequences with 3,070 ms repetition time and 63.8 ms echo time. The acquired PET images were reconstructed with the 3 dimensional ordered subset expectation maximization algorithm.
• Acquired PET and MRI images were spatially registered for the FDG standard uptake value (SUV) evaluation in the tumor sites.
Image and statistical analyses
Histology and immunohistochemistry (IHC)
RESULTS
Selection of optimal tumor size and dose for [18F]-FDG-PET imaging protocol
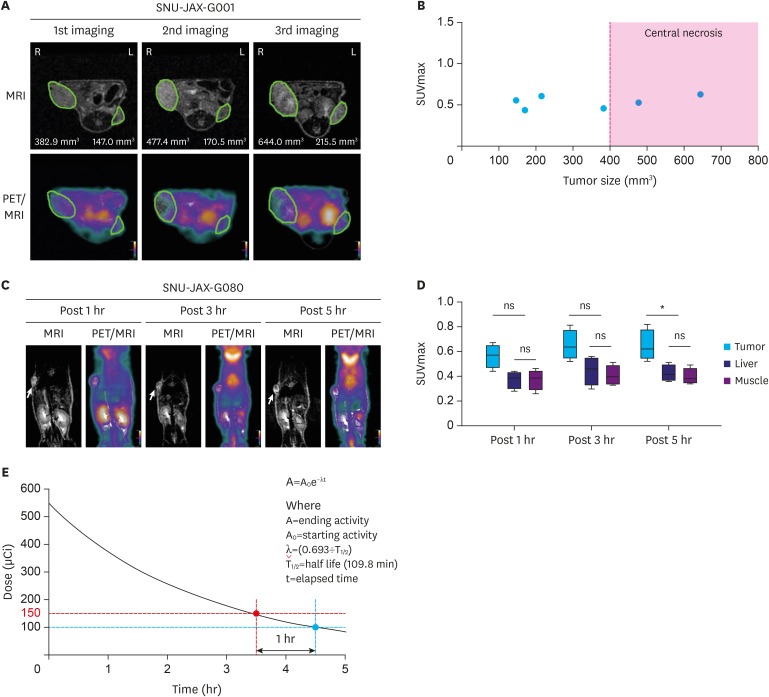 | Fig. 2Selection of optimal tumor size and dose for [18F]-FDG-PET imaging protocol. (A) Serial PET/MRI images in a heterotopic model at 54 (1st imaging), 61 (2nd imaging), and 72 (3rd imaging) days after modeling. The green ellipsoid indicates a tumor. (B) Evaluation of [18F]-FDG uptake in different sized tumors. (C) Consecutive PET/MRI images of mice bearing heterotopic PDX tumor 1, 3, and 5 hours following [18F]-FDG injection (n=4). The yellow arrow indicates a tumor. (D) [18F]-FDG uptake in tumors and normal background tissues. Box plots with error bars indicate the mean uptake and standard deviation across the mice. (E) Injection dose selection for PET imaging protocol from the theoretical decay curve of F-18.[18F]-FDG-PET = 18-fluordesoxyglucose positron emission tomography; PET = positron emission tomography; MRI = magnetic resonance imaging; PDX = patient-derived xenograft; SUVmax = maximal standardized uptake value; ns = not significant.
*P-value ≤0.05.
|
Inflammatory signal aspect for orthotopic model
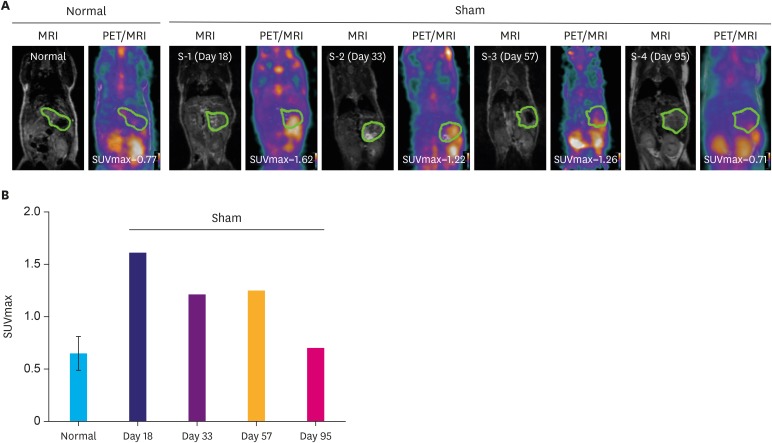 | Fig. 3Inflammatory PET signal aspect for orthotopic model. (A) [18F]-FDG-PET/MRI images of normal (n=2) and sham mouse models. The green ellipsoid indicates the stomach. (B) Quantitative analysis of FDG uptake using SUVmax in stomach.[18F]-FDG-PET = 18-fluordesoxyglucose positron emission tomography; PET = positron emission tomography; MRI = magnetic resonance imaging; FDG = fluordesoxyglucose; SUVmax = maximal standardized uptake value.
|
Comparison of corresponding PDX tissue in heterotopic and orthotopic models
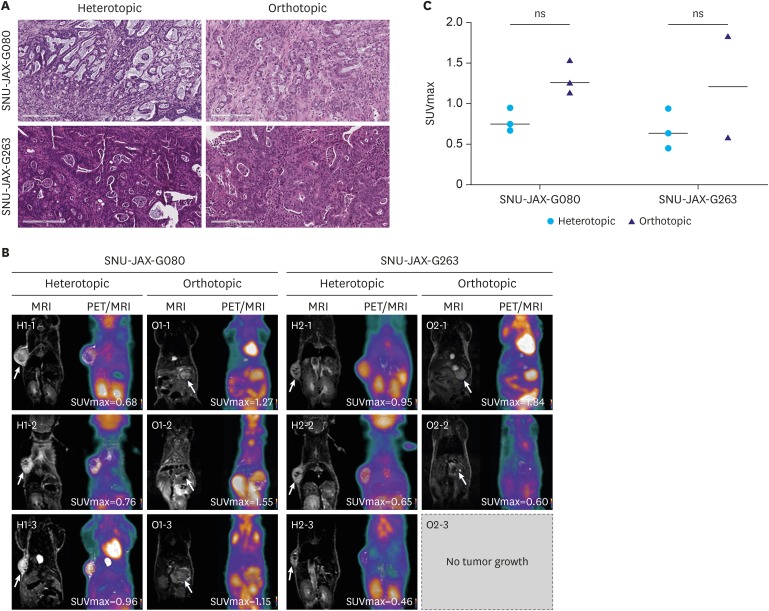 | Fig. 4Comparison of corresponding PDX tissue in heterotopic and orthotopic models. (A) Representative H&E staining images of tumor growth in established models (H&E stain, ×200). (B) [18F]-FDG-PET/MRI images of heterotopic and orthotopic mouse models. (C) Quantitative analysis of [18F]-FDG uptake using SUVmax in heterotopic and orthotopic models bearing PDX tumor.PDX = patient-derived xenograft; H&E = hematoxylin and eosin; [18F]-FDG-PET = 18-fluordesoxyglucose positron emission tomography; PET = positron emission tomography; MRI = magnetic resonance imaging; FDG = fluordesoxyglucose; SUVmax = maximal standardized uptake value; ns = not significant.
|
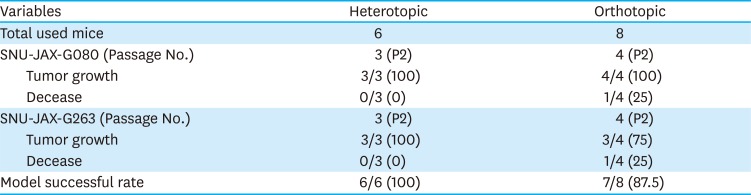
Table 2
Summary of successful rate between heterotopic and orthotopic models
Expression levels of GLUT1 and HK2 in PET-scanned tumors
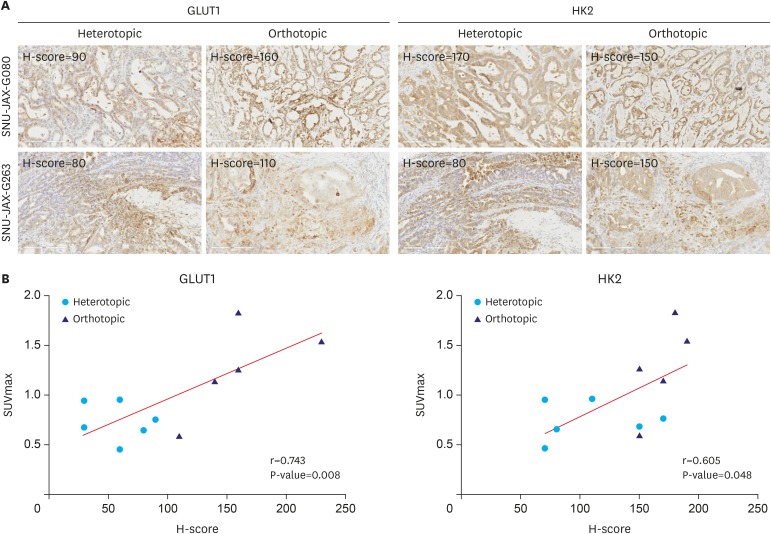 | Fig. 5Correlation between FDG uptake and glycolysis-related protein levels. (A) Representative immunohistochemical results of GLUT1 and HK2 in PET-scanned tumors (IHC stain, ×200). (B) Comparative analysis between [18F]-FDG uptake and immunohistochemical staining score.FDG = fluordesoxyglucose; GLUT1 = glucose transporter 1; HK2 = hexokinase 2; PET = positron emission tomography; IHC = immunohistochemistry; [18F]-FDG = 18-fluordesoxyglucose; MRI = magnetic resonance imaging; SUVmax = maximal standardized uptake value.
Pearson r=0.743, P-value <0.01 and Pearson r=0.605, P-value <0.05 for GLUT1 and HK2, respectively.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download