1. Takahashi T, Sawai K, Hagiwara A, Takahashi S, Seiki K, Tokuda H. Type-oriented therapy for gastric cancer effective for lymph node metastasis: management of lymph node metastasis using activated carbon particles adsorbing an anticancer agent. Semin Surg Oncol. 1991; 7:378–383. PMID:
1759087.

2. Coller FA, Kay EB, McIntyre RS. Regional lymphatic metastases of carcinoma of the stomach. Arch Surg. 1941; 43:748–761.

3. Pissas A, Sarrazin R, Dyon JF, Bouchet Y. The lymphatic vessels of the stomach in man. Folia Morphol (Praha). 1982; 30:363–365. PMID:
7160801.
4. Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg. 1987; 11:418–425. PMID:
3630186.

5. Son T, Hyung WJ, Kim JW, Kim HI, An JY, Cheong JH, et al. Anatomic extent of metastatic lymph nodes: still important for gastric cancer prognosis. Ann Surg Oncol. 2014; 21:899–907. PMID:
24276641.

6. Choi YY, An JY, Guner A, Kang DR, Cho I, Kwon IG, et al. Skip lymph node metastasis in gastric cancer: is it skipping or skipped? Gastric Cancer. 2016; 19:206–215. PMID:
25708370.

7. Sasada S, Ninomiya M, Nishizaki M, Harano M, Ojima Y, Matsukawa H, et al. Frequency of lymph node metastasis to the splenic hilus and effect of splenectomy in proximal gastric cancer. Anticancer Res. 2009; 29:3347–3351. PMID:
19661354.
8. Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981; 11:127–139. PMID:
7300058.
9. Maruyama K, Gunvén P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg. 1989; 210:596–602. PMID:
2818028.
10. Okajima K, Isozaki H. Splenectomy for treatment of gastric cancer: Japanese experience. World J Surg. 1995; 19:537–540. PMID:
7676696.

11. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English edition -. Gastric Cancer. 1998; 1:10–24. PMID:
11957040.
12. Mönig SP, Collet PH, Baldus SE, Schmackpfeffer K, Schröder W, Thiele J, et al. Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol. 2001; 76:89–92. PMID:
11223832.
13. Ooki A, Yamashita K, Kikuchi S, Sakuramoto S, Katada N, Hutawatari N, et al. Clinical significance of total gastrectomy for proximal gastric cancer. Anticancer Res. 2008; 28:2875–2883. PMID:
19031928.
14. Shin SH, Jung H, Choi SH, An JY, Choi MG, Noh JH, et al. Clinical significance of splenic hilar lymph node metastasis in proximal gastric cancer. Ann Surg Oncol. 2009; 16:1304–1309. PMID:
19241107.

15. Aoyagi K, Kouhuji K, Miyagi M, Imaizumi T, Kizaki J, Shirouzu K. Prognosis of metastatic splenic hilum lymph node in patients with gastric cancer after total gastrectomy and splenectomy. World J Hepatol. 2010; 2:81–86. PMID:
21160977.

16. Kosuga T, Ichikawa D, Okamoto K, Komatsu S, Shiozaki A, Fujiwara H, et al. Survival benefits from splenic hilar lymph node dissection by splenectomy in gastric cancer patients: relative comparison of the benefits in subgroups of patients. Gastric Cancer. 2011; 14:172–177. PMID:
21331530.

17. Zhu GL, Sun Z, Wang ZN, Xu YY, Huang BJ, Xu Y, et al. Splenic hilar lymph node metastasis independently predicts poor survival for patients with gastric cancers in the upper and/or the middle third of the stomach. J Surg Oncol. 2012; 105:786–792. PMID:
22105768.

18. Chen XL, Yang K, Zhang WH, Chen XZ, Zhang B, Chen ZX, et al. Metastasis, risk factors and prognostic significance of splenic hilar lymph nodes in gastric adenocarcinoma. PLoS One. 2014; 9:e99650. PMID:
24915065.

19. Yang K, Zhang WH, Chen XZ, Chen XL, Zhang B, Chen ZX, et al. Survival benefit and safety of no. 10 lymphadenectomy for gastric cancer patients with total gastrectomy. Medicine (Baltimore). 2014; 93:e158. PMID:
25437029.

20. Sun Z, Wang Q, Yu X, Ou C, Yao L, Liu K, et al. Risk factors associated with splenic hilar lymph node metastasis in patients with advanced gastric cancer in northwest China. Int J Clin Exp Med. 2015; 8:21358–21364. PMID:
26885077.
21. Hong ZL, Chen QY, Zheng CH, Li P, Xie JW, Wang JB, et al. A preoperative scoring system to predict the risk of No.10 lymph node metastasis for advanced upper gastric cancer: a large case report based on a single-center study. Oncotarget. 2017; 8:80050–80060. PMID:
29108387.

22. Watanabe M, Kinoshita T, Enomoto N, Shibasaki H, Nishida T. Clinical significance of splenic hilar dissection with splenectomy in advanced proximal gastric cancer: an analysis at a single institution in Japan. World J Surg. 2016; 40:1165–1171. PMID:
26630939.

23. Son T, Kwon IG, Lee JH, Choi YY, Kim HI, Cheong JH, et al. Impact of splenic hilar lymph node metastasis on prognosis in patients with advanced gastric cancer. Oncotarget. 2017; 8:84515–84528. PMID:
29137444.

24. Maezawa Y, Aoyama T, Yamada T, Kano K, Hayashi T, Sato T, et al. Priority of lymph node dissection for proximal gastric cancer invading the greater curvature. Gastric Cancer. 2018; 21:569–572. PMID:
29119277.

25. Jeong O, Jung MR, Ryu SY. Clinicopathological features and prognostic impact of splenic hilar lymph node metastasis in proximal gastric carcinoma. Eur J Surg Oncol. 2019; 45:432–438. PMID:
30389304.

26. Yura M, Yoshikawa T, Otsuki S, Yamagata Y, Morita S, Katai H, et al. The therapeutic survival benefit of splenic hilar nodal dissection for advanced proximal gastric cancer invading the greater curvature. Ann Surg Oncol. 2019; 26:829–835. PMID:
30569298.

27. Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995; 82:346–351. PMID:
7796005.

28. Nashimoto A, Yabusaki H, Matsuki A. The significance of splenectomy for advanced proximal gastric cancer. Int J Surg Oncol. 2012; 2012:301530. PMID:
22685639.

29. Yura M, Yoshikawa T. ASO author reflections: splenic hilar nodal dissection for proximal advanced gastric cancer. Ann Surg Oncol. 2019; 26:588–589. PMID:
31016488.
30. Csendes A, Burdiles P, Rojas J, Braghetto I, Diaz JC, Maluenda F. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery. 2002; 131:401–407. PMID:
11935130.

31. Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. 2017; 265:277–283. PMID:
27280511.

32. Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008; 359:453–462. PMID:
18669424.

33. Kinoshita T. Splenic hilar dissection in the treatment of proximal advanced gastric cancer: what is an adequate strategy? Transl Gastroenterol Hepatol. 2016; 1:72. PMID:
28138638.

34. Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg. 2006; 93:559–563. PMID:
16607678.

35. Hyung WJ, Lim JS, Song J, Choi SH, Noh SH. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg. 2008; 207:e6–e11. PMID:
18656040.

36. Huang CM, Chen QY, Lin JX, Zheng CH, Li P, Xie JW, et al. Laparoscopic spleen-preserving splenic hilar lymphadenectomy performed by following the perigastric fascias and the intrafascial space for advanced upper-third gastric cancer. PLoS One. 2014; 9:e90345. PMID:
24603610.

37. Ji X, Fu T, Bu ZD, Zhang J, Wu XJ, Zong XL, et al. Comparison of different methods of splenic hilar lymph node dissection for advanced upper- and/or middle-third gastric cancer. BMC Cancer. 2016; 16:765. PMID:
27716191.

38. Huang CM, Huang ZN, Zheng CH, Li P, Xie JW, Wang JB, et al. Huang's three-step maneuver shortens the learning curve of laparoscopic spleen-preserving splenic hilar lymphadenectomy. Surg Oncol. 2017; 26:389–394. PMID:
29113657.

39. Guo X, Peng Z, Lv X, Cui J, Zhang K, Li J, et al. Randomized controlled trial comparing short-term outcomes of laparoscopic and open spleen-preserving splenic hilar lymphadenectomy for advanced proximal gastric cancer: an interim report. J Surg Oncol. 2018; 118:1264–1270. PMID:
30380145.

40. Yang K, Cho M, Roh CK, Seo WJ, Choi S, Son T, et al. Robotic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. Surg Endosc. 2019; 33:2357–2363. PMID:
30945060.

41. Son T, Lee JH, Kim YM, Kim HI, Noh SH, Hyung WJ. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc. 2014; 28:2606–2615. PMID:
24695982.

42. Wang JB, Huang CM, Zheng CH, Li P, Xie JW, Lin JX, et al. Role of 3DCT in laparoscopic total gastrectomy with spleen-preserving splenic lymph node dissection. World J Gastroenterol. 2014; 20:4797–4805. PMID:
24782634.

43. Kinoshita T, Shibasaki H, Enomoto N, Sahara Y, Sunagawa H, Nishida T. Laparoscopic splenic hilar lymph node dissection for proximal gastric cancer using integrated three-dimensional anatomic simulation software. Surg Endosc. 2016; 30:2613–2619. PMID:
26310530.

44. Kwon IG, Son T, Kim HI, Hyung WJ. Fluorescent lymphography-guided lymphadenectomy during robotic radical gastrectomy for gastric cancer. JAMA Surg. 2019; 154:150–158. PMID:
30427990.

45. Kim YM, Baek SE, Lim JS, Hyung WJ. Clinical application of image-enhanced minimally invasive robotic surgery for gastric cancer: a prospective observational study. J Gastrointest Surg. 2013; 17:304–312. PMID:
23207683.

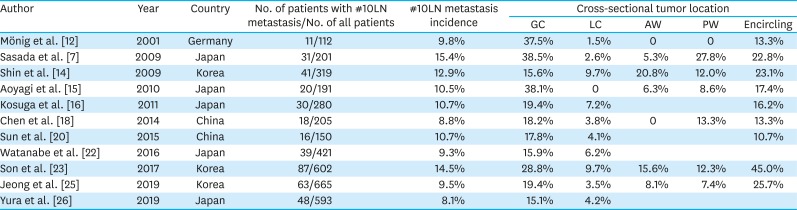
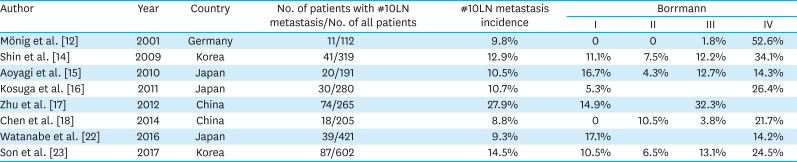
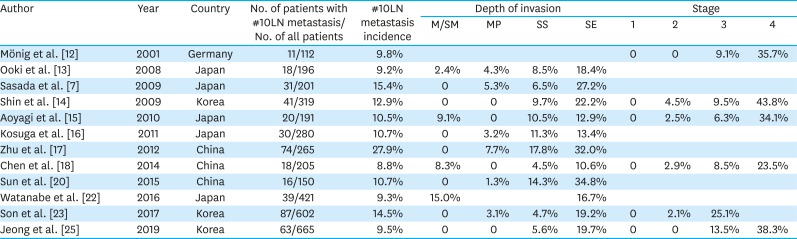




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download