1. Buisman FE, van Gelder L, Menke-Pluijmers MB, Bisschops BH, Plaisier PW, Westenend PJ. Non-primary breast malignancies: a single institution's experience of a diagnostic challenge with important therapeutic consequences-a retrospective study. World J Surg Oncol. 2016; 14:166. PMID:
27337944.

2. Lee SK, Kim WW, Kim SH, Hur SM, Kim S, Choi JH, et al. Characteristics of metastasis in the breast from extramammary malignancies. J Surg Oncol. 2010; 101:137–140. PMID:
20082359.

3. Georgiannos SN, Chin J, Goode AW, Sheaff M. Secondary neoplasms of the breast: a survey of the 20th Century. Cancer. 2001; 92:2259–2266. PMID:
11745279.
4. Williams SA, Ehlers RA 2nd, Hunt KK, Yi M, Kuerer HM, Singletary SE, et al. Metastases to the breast from nonbreast solid neoplasms: presentation and determinants of survival. Cancer. 2007; 110:731–737. PMID:
17582626.
5. Sun P, Chen J, Lu J, Luo R, Li M, He J. Characteristics of breast metastases from non-breast solid tumors in 22 patients from a southern Chinese population. Oncol Lett. 2018; 15:3685–3693. PMID:
29467888.
6. DeLair DF, Corben AD, Catalano JP, Vallejo CE, Brogi E, Tan LK. Non-mammary metastases to the breast and axilla: a study of 85 cases. Mod Pathol. 2013; 26:343–349. PMID:
23174933.

7. Ali RH, Taraboanta C, Mohammad T, Hayes MM, Ionescu DN. Metastatic non-small cell lung carcinoma a mimic of primary breast carcinoma-case series and literature review. Virchows Arch. 2018; 472:771–777. PMID:
29105026.

8. Alva S, Shetty-Alva N. An update of tumor metastasis to the breast data. Arch Surg. 1999; 134:450. PMID:
10199322.

9. Ma Y, Liu W, Li J, Xu Y, Wang H. Gastric cancer with breast metastasis: Clinical features and prognostic factors. Oncol Lett. 2018; 16:5565–5574. PMID:
30344710.

10. Amin MB, editor. AJCC Cancer Staging Manual. 8th ed. Chicago (IL): Springer;2017. p. 207–220.
11. Lauwers GY, Carneiro F, Craham DY. Gastric carcinoma. In : Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press;2010. p. 45–58.
12. Bartley AN, Washington MK, Ventura CB, Ismaila N, Colasacco C, Benson AB 3rd, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Arch Pathol Lab Med. 2016; 140:1345–1363. PMID:
27841667.

13. Hissong E, Ramrattan G, Zhang P, Zhou XK, Young G, Klimstra DS, et al. Gastric carcinoma with lymphoid stroma. An evaluation of the histopathologic and molecular features. Am J Surg Pathol. 2018; 42:453–462. PMID:
29438172.
14. Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan AH, Matsuo K, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014; 63:236–243. PMID:
23580779.

15. Vanoli A, Di Sabatino A, Martino M, Dallera E, Furlan D, Mescoli C, et al. Epstein-Barr virus-positive ileal carcinomas associated with Crohn's disease. Virchows Arch. 2017; 471:549–552. PMID:
28752215.

16. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014; 513:202–209. PMID:
25079317.
17. Saito R, Abe H, Kunita A, Yamashita H, Seto Y, Fukayama M. Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1
+ immune cells in Epstein-Barr virus-associated gastric cancer: the prognostic implications. Mod Pathol. 2017; 30:427–439. PMID:
27934877.
18. De Rosa S, Sahnane N, Tibiletti MG, Magnoli F, Vanoli A, Sessa F, et al. EBV
+ and MSI gastric cancers harbor high PD-L1/PD-1 expression and high CD8
+ intratumoral lymphocytes. Cancers (Basel). 2018; 10:E102. PMID:
29614789.
19. Nayar M, Chandra M, Aggarwal R, Chander S. Carcinoma cervix presenting as primary breast malignancy. Indian J Pathol Microbiol. 1987; 30:283–286. PMID:
2835316.
20. Jayi S, Fatemi H, Laabadi K, Bouguern H, Chaara H, Laamarti A, et al. Breast metastases of gastric adenocarcinoma associated with a pregnancy of 32 weeks of gestation: about a rare case. Pan Afr Med J. 2013; 15:116–122. PMID:
24255722.

21. Song MJ, Park YS, Song HJ, Park SJ, Ahn JY, Choi KD, et al. Prognosis of pregnancy-associated gastric cancer: and age-, sex-, and stage-matched case-control study. Gut Liver. 2016; 10:731–738. PMID:
27114414.
22. Peccatori FA, Azim HA Jr, Orecchia R, Hoekstra HJ, Pavlidis N, Kesic V, et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24 Suppl 6:vi160–vi170. PMID:
23813932.

23. Al-Ibrahim A, Parrish J, Dunn E, Swallow C, Maxwell C. Pregnancy and maternal outcomes in women with prior or current gastrointestinal malignancies. J Obstet Gynaecol Can. 2014; 36:34–41. PMID:
24444285.

24. Saito M, Okayama H, Saito K, Ando J, Kumamoto K, Nakamura I, et al. CDX2 is involved in microRNA-associated inflammatory carcinogenesis in gastric cancer. Oncol Lett. 2017; 14:6184–6190. PMID:
29113265.

25. Hirano N, Tsukamoto T, Mizoshita T, Koriyama C, Akiba S, Campos F, et al. Down regulation of gastric and intestinal phenotypic expression in Epstein-Barr virus-associated stomach cancers. Histol Histopathol. 2007; 22:641–649. PMID:
17357094.
26. Shet T, Pai T, Shetty O, Desai S. Lymphoepithelioma-like carcinoma of breast-evaluation for Epstein-Barr virus-encoded RNA, human papillomavirus, and markers of basal cell differentiation. Ann Diagn Pathol. 2016; 25:42–47. PMID:
27806845.

27. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Van de Vijver MJ. WHO Classification of Tumours of the Breast. Lyon: IARC Press;2012. p. 46–47.
28. Glaser SL, Hsu JL, Gulley ML. Epstein-Barr virus and breast cancer: state of the evidence for viral carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2004; 13:688–697. PMID:
15159298.

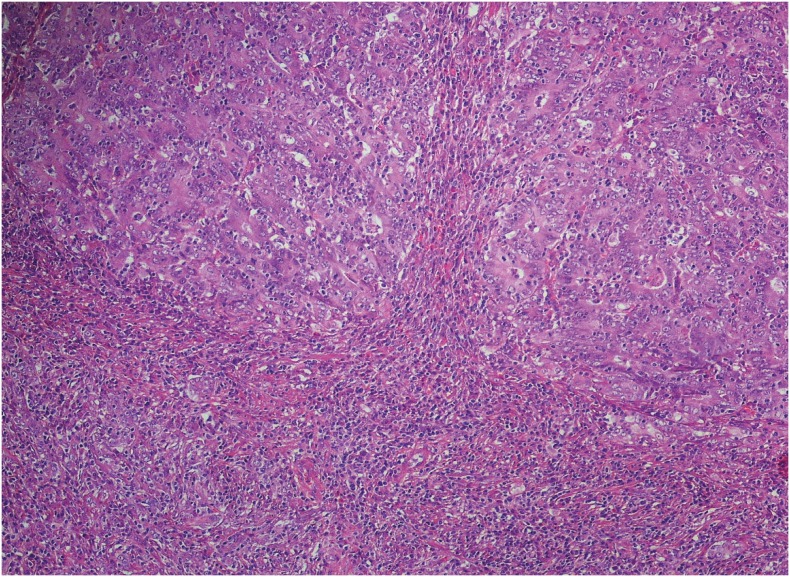
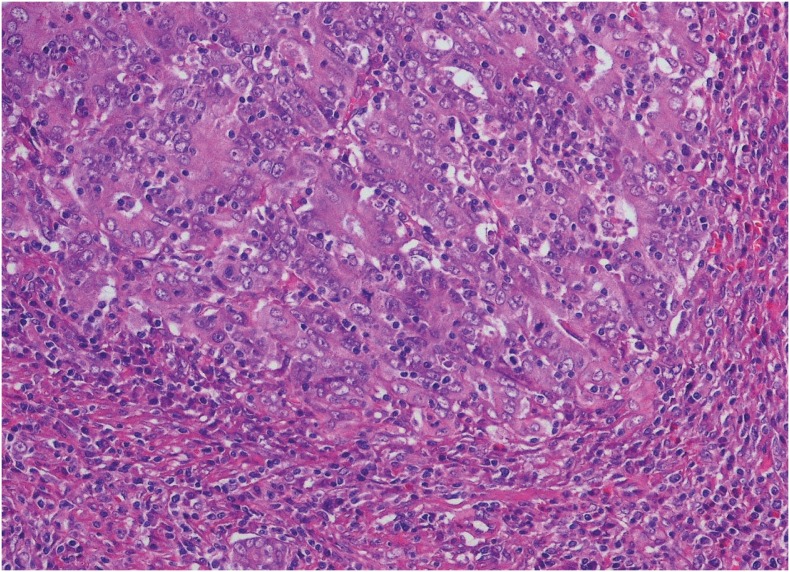
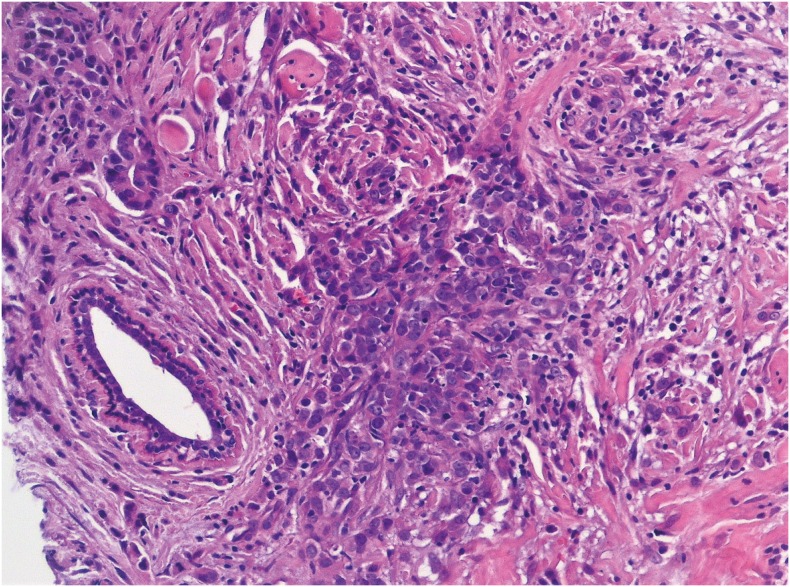
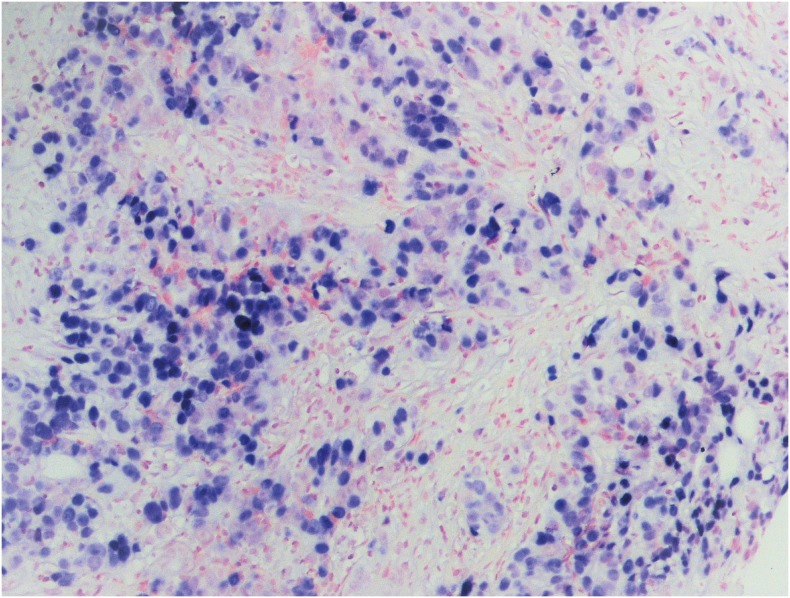




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download