Abstract
Purpose
Materials and Methods
Results
Conclusion
Figures and Tables
 | Fig. 1Diagram of the study design. OA, osteoarthritis; GH, growth hormone; PBS, phosphate buffered saline; CT, micro-computed tomography. |
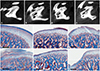 | Fig. 2OA scoring of the bone (micro-CT) and cartilage (histologic finding). (A-D) Central sagittal micro-CT images of erosive condyles to score subchondral bone: bony OA score=0 (A , None), 1 (B, Minor), 2 (C, Moderate), and 3 (D, Severe). (E-J) Histologic findings (Masson's trichrome staining): healthy (E), cartilaginous OA score=1 (F), 2 (G), 3 (H), 4 (I), and 5 (J). The black scale bar in the histological images represents 300 µm. OA, osteoarthritis; CT, computed tomography. |
 | Fig. 3GH injections into the TMJ increase serum GH levels, but not IGF-1 levels. After two and four injections of PBS(Rt) or GH(Lt) into the TMJ, GH (A) and IGF-1 (B) concentrations in serum were measured using ELISA . Healthy (n=2), OA-induced only (n=5, allowed to self-heal after induction of OA), and OA+GH injected (n=10). *p=0.003 (Mann-Whitney test). GH, growth hormone; IGF-1, insulin-like growth factor 1; PBS, phosphate buffered saline; TMJ, temporomandibular joint; ELISA, enzyme-linked immunosorbent assay; OA, osteoarthritis. |
 | Fig. 4GH injections into the TMJ increase IGF-1 levels in synovial fluid. After two and four injections of PBS(Rt) or GH(Lt) into the TMJ, the GH (A) and IGF-1 (B) concentrations in synovial fluid from the TMJ were measured by ELISA. OA-induced only (n=5, allowed to self-heal after induction of OA), OA+PBS injected (n=10), and OA+GH injected (n=10). *p=0.016 (Kruskal-Wallis test). GH, growth hormone; TMJ, temporomandibular joint; IGF-1, insulin-like growth factor 1; PBS, phosphate buffered saline; ELISA, enzyme-linked immunosorbent assay; OA, osteoarthritis. |
 | Fig. 5Local injection of GH ameliorates OA joint in subchondral bone of the temporomandibular. OA scores were determined using in vivo micro-CT images performed before PBS or GH administration and after two (A) and four (B) injections of PBS or GH. OA-induced only (n=5, allowed to self-heal after induction of OA), OA+PBS injected (n=10), and OA+GH injected (n=10). *Differences were statistically significant at p<0.05, †Differences were statistically significant at p<0.01. OA, osteoarthritis; CT, computed tomography; CT1, OA score observed on initial CT images immediately after OA was induced; CT2, OA score observed on CT images after two injections; CT3, OA score observed on CT images after four injections; GH, growth hormone; TMJ-OA, osteoarthritis of the temporomandibular joint; micro-CT, micro-computed tomography; PBS, phosphate buffered saline. |
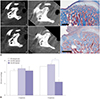 | Fig. 6GH injection into the MIA-induced OA of the TMJ area significantly improves OA scores in the cartilage. (A) All images were obtained from one rat after four injections of GH in the left TMJ (a, b, c) and PBS in the right TMJ (d, e, f). TMJ-OA was induced by MIA injection, and micro-CT images (CT1) were acquired at 2 weeks after MIA injection to confirm OA induction (a, d). Treatment with GH (b) or PBS (e) injection was administered four times, and images (CT3) were taken after administration. Histologic findings of panels (b) and (e) were observed using Masson's trichrome stain respective to (c) and (f). The black scale bar in the histological images represents 500 µm. (B) Histologic OA scores were compared between PBS- and GH-injected groups. OA-induced only (n=5, allowed to self-heal after induction of OA), OA+PBS injected (n=10), and OA+GH injected (n=10). *p=0.004 (Mann-Whitney test). OA, osteoarthritis; GH, growth hormone; MIA, monosodium iodoacetate; TMJ-OA, osteoarthritis of the temporomandibular joint; micro-CT, micro-computed tomography; PBS, phosphate buffered saline. |
ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: Sung-Hee Jeong.
Data curation: Jin-Hwa Kim, Ji- Su Kim, and Eun-gyo Jeong.
Formal analysis: Sun-Nyoung Yu.
Funding acquisition: Sung-Hee Jeong.
Investigation: Sun-Nyoung Yu.
Methodology: Yang Mi Park.
Project administration: Hye-Mi Jeon, Yong-Woo Ahn, and Kyung-Hee Kim.
Resources: Jun-Young Heo.
Software: Jin-Hwa Kim and Eun-gyo Jeong.
Supervision: Sung-Hee Jeong.
Validation: Hae Ryoun Park.
Visualization: Sun-Nyoung Yu.
Writing—original draft: Sung-Hee Jeong and Soo-Min Ok.
Writing—review & editing: Sung-Hee Jeong, Soo-Min Ok, Soon-Cheol Ahn, and Hae Ryoun Park.
Approval of final manuscript: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download