Abstract
Coccidiosis-causing Eimeria species are transmitted in poultry via the oral-fecal route and can lead to hemorrhagic diarrhea and mortality. This results in enormous economic losses in the poultry industry. Furthermore, its resistance to some currently used antibiotics is increasing. This has prompted the development of new alternative drug therapies that address the issue of chemical-free meat production. Effective management of infectious diseases in veterinary practice includes the induction of protective and adaptive immunity by treatment with an alternative agent. In this study, we evaluated the anticoccidial effects of dietary supplementation of Chosun University (CS) 32 compounds (0.1% and 1.0%) against Eimeria tenella, which was isolated and purified from the supernatant of culture broth of Bacillus strain (KCTC18250P), as well as its effect on the growth rate and feed efficiency in chickens. Overall, we observed a decrease in lesion scores and oocyte output in CS 32 compounds-treated chickens. We concluded that 0.1% CS 32 compounds displayed anticoccidial effects against E. tenella infection.
Go to : 
REFERENCES
1). McDonald V, Shirley MW. Past and future: vaccination against Eimeria. Parasitology. 2009; 136:1477–89.
2). Sharman PA, Smith NC, Wallach MG, Katrib M. Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol. 2010; 32:590–8.

3). Del Cacho E, Gallego M, Lillehoj HS, Quí lez J, Lillehoj EP, Ramo A, et al. IL-17A regulates Eimeria tenella schizont maturation and migration in avian coccidiosis. Vet Res. 2014; 45:25.
4). Peek HW, Landman WJ. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet Q. 2011; 31:143–61.

5). Choi YH, Cho SS, Simkhada JR, Rahman MS, Choi YS, Kim CS, et al. A novel multifunctional peptide oligomer of bacitracin with possible bioindustrial and therapeutic applications from a Korean food-source Bacillus strain. PloS ONE. 2017; 12:e0176971.
6). Kim SE, Choi GH, Shim KM, Kim JC, Yo JC, Cho SS, et al. Effects of probiotic CS-32 as a feed additive on growth performance of broiler chickens. J Biomed Res. 2013; 14:170–6.

7). Lee SB, Kim BK, Park CH, Park GH, Jin YC, Kang HS, et al. Effects of dietary probiotics and immunomodulator as an alternative to antibiotics in Korean Native Chicken. J Anim Sci Technol. 2011; 53:409–18.

8). Jeong J, Kim WH, Yoo J, Lee C, Kim S, Cho JH, et al. Identification and comparative expression analysis of interleukin 2/15 receptor beta chain in chickens infected with E. tenella. PLoS One. 2012; 7:e37704.
9). Chapman HD, Sandstrom J, Breeding SW. Effect of the anticoccidial agents halofuginone and monensin when given with growth promoting antibiotics upon the control of coccidiosis in the turkey. Avian Pathol. 1998; 27:498–504.

10). Johnson J, Reid WM. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970; 28:30–6.

11). Chu GM, Lee SJ, Jeong HS, Lee SS. Efficacy of probiotics from anaerobic microflora with prebiotics on growth performance and noxious gas emission in growing pigs. Anim Sci J. 2011; 82:282–90.
12). Mookiah S, Sieo CC, Ramasamy K, Abdullah N, Ho YW. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J Sci Food Agric. 2014; 94:341–8.

13). Mountzouris KC, Tsitrsikos P, Palamidi I, Arvaniti A, Mohnl M, Schatzmayr G, et al. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult Sci. 2010; 89:58–67.

14). Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989; 66:365–78.
16). Ko SY, Bae IH, Yee ST, Lee SS, Uuganbayar D, Oh JI, et al. Comparison of the effect of green tea by-product and green tea probiotics on the growth performance, meat quality, and immune response of finishing pigs. Asian-Aust J Anim Sci. 2008; 21:1486–94.

17). Ko SY, Yang CJ. Effect of green tea probiotics on the growth performance, meat quality and immune response in finishing pigs. Asian-Aust J Anim Sci. 2008; 21:1339–47.

18). Mohan B, Kadirvel R, Natarajan A, Bhaskaran M. Effect of probiotic supplementation on growth, nitrogen utilisation and serum cholesterol in broilers. Br Poult Sci. 1996; 37:395–401.

19). Taylor CC, Ranjit NJ, Mills JA, Neylon JM, Kung L Jr. The effect of treating whole-plant barley with Lactobacillus buchneri 40788 on silage fermentation, aerobic stability, and nutritive value for dairy cows. J Dairy Sci. 2002; 85:1793–800.
20). Silo-Suh LA, Lethbridge BJ, Raffel SJ, He H, Clardy J, Handelsman J. Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85. Appl Environ Microbiol. 1994; 60:2023–30.
21). Katz E, Demain AL. The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriol Rev. 1977; 41:449–74.
22). Amin A, Khan MA, Ehsanullah M, Haroon U, Azam SM, Hameed A. Production of peptide antibiotics by Bacillus sp. GU 057 indigenously isolated from saline soil. Braz J Microbiol. 2012; 43:1340–6.
23). Sengupta S, Banerjee A, Bose SK. γ-Glutamyl and D-or L-peptide linkages in mycobacillin, a cyclic peptide antibiotic. Biochem J. 1971; 121:839–46.
24). Wakayama S, Ishikawa F, Oishi K. Mycocerein, a novel antifungal peptide antibiotic produced by Bacillus cereus. Antimicrob Agents Chemother. 1984; 26:939–40.
25). Isogai A, Takayama S, Murakoshi S, Suzuki A. Structure of β-amino acids in antibiotics iturin A. Tetrahedron Lett. 1982; 23:3065–8.

26). Peypoux F, Besson F, Michel G, Delcambe L. Structure of bacillomycin D, a new antibiotic of the iturin group. Eur J Biochem. 1981; 118:323–7.

27). Dion HW, Woo PW, Willmer NE, Kern DL, Onaga J, Fusari SA. Butirosin, a new aminoglycosidic antibiotic complex: isolation and characterization. Antimicrob Agents Chemother. 1972; 2:84–8.

28). Horii S, Nogami I, Mizokami N, Arai Y, Yoneda M. New antibiotic produced by bacteria, 5-β-D-xylofuranosylneamine. Antimicrob Agents Chemother. 1974; 5:578–81.
29). Reid WM, Johnson J, Dick J. Anticoccidial activity of lasalocid in control of moderate and severe coccidiosis. Avian Dis. 1975; 19:12–8.

30). Tewari AK, Maharana BR. Control of poultry coccidiosis: changing trends. J Parasit Dis. 2011; 35:10–7.

31). Levine R, Horst G, Tonda R, Lumpkins B, Mathis G. Evaluation of the effects of feeding dried algae containing beta-1, 3-glucan on broilers challenged with Eimeria. Poult Sci. 2018; 97:3494–500.
Go to : 
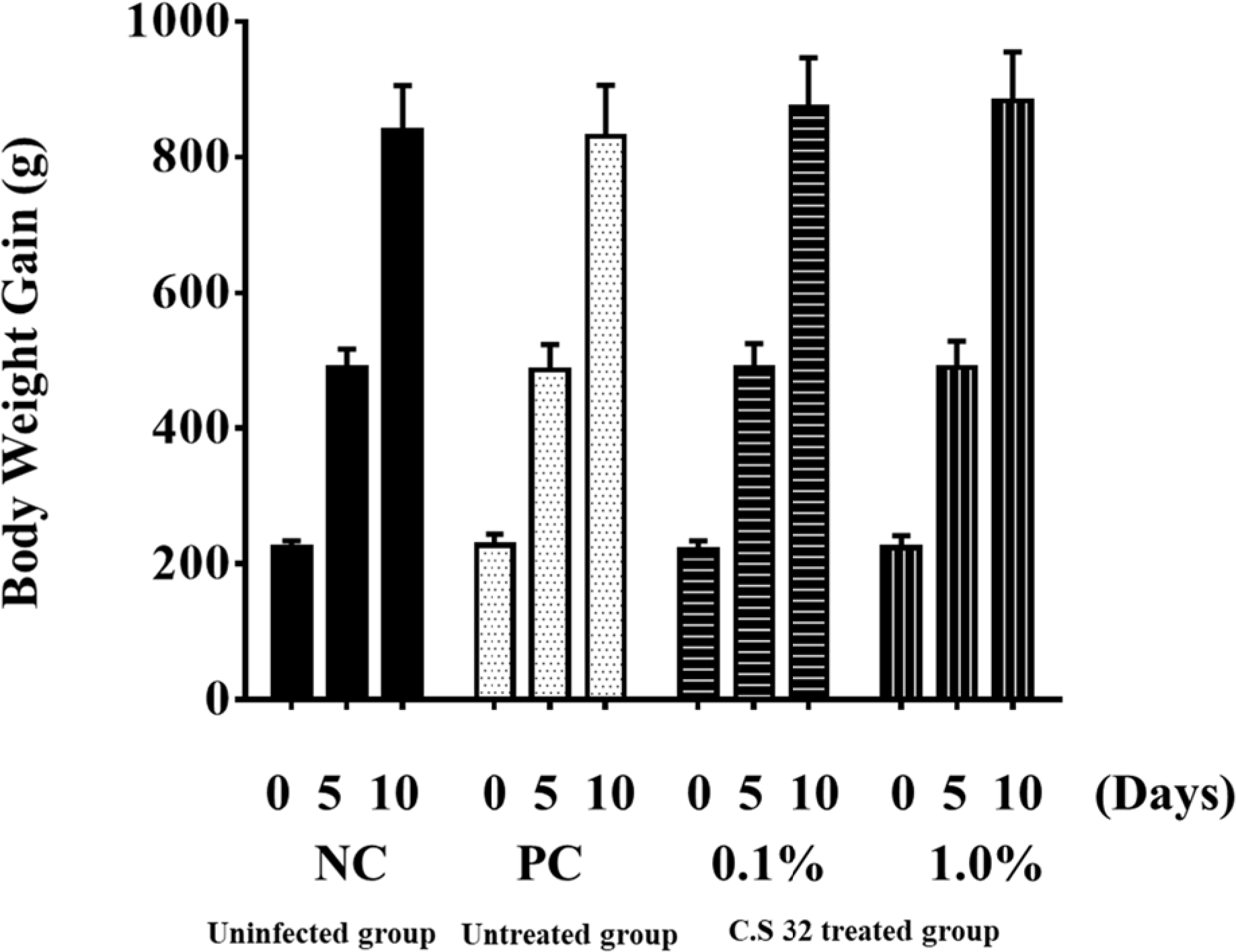 | Fig. 1.Effects of dietary supplementation with CS 32 compounds on weight gains after challenged with E tenella. NC: neg ative control (uninfected and untreated group); PC: positive control (infected and untreated group); 0.1%: 0.1% CS 32 co impounds group (infected and treated with 0.1% CS 32 compounds); 1.0%: 1.0% CS 32 compounds group (infected and treated with 1.0% CS 32 compounds); n=28 for days 0 and 5, and n=21 for days 10. |
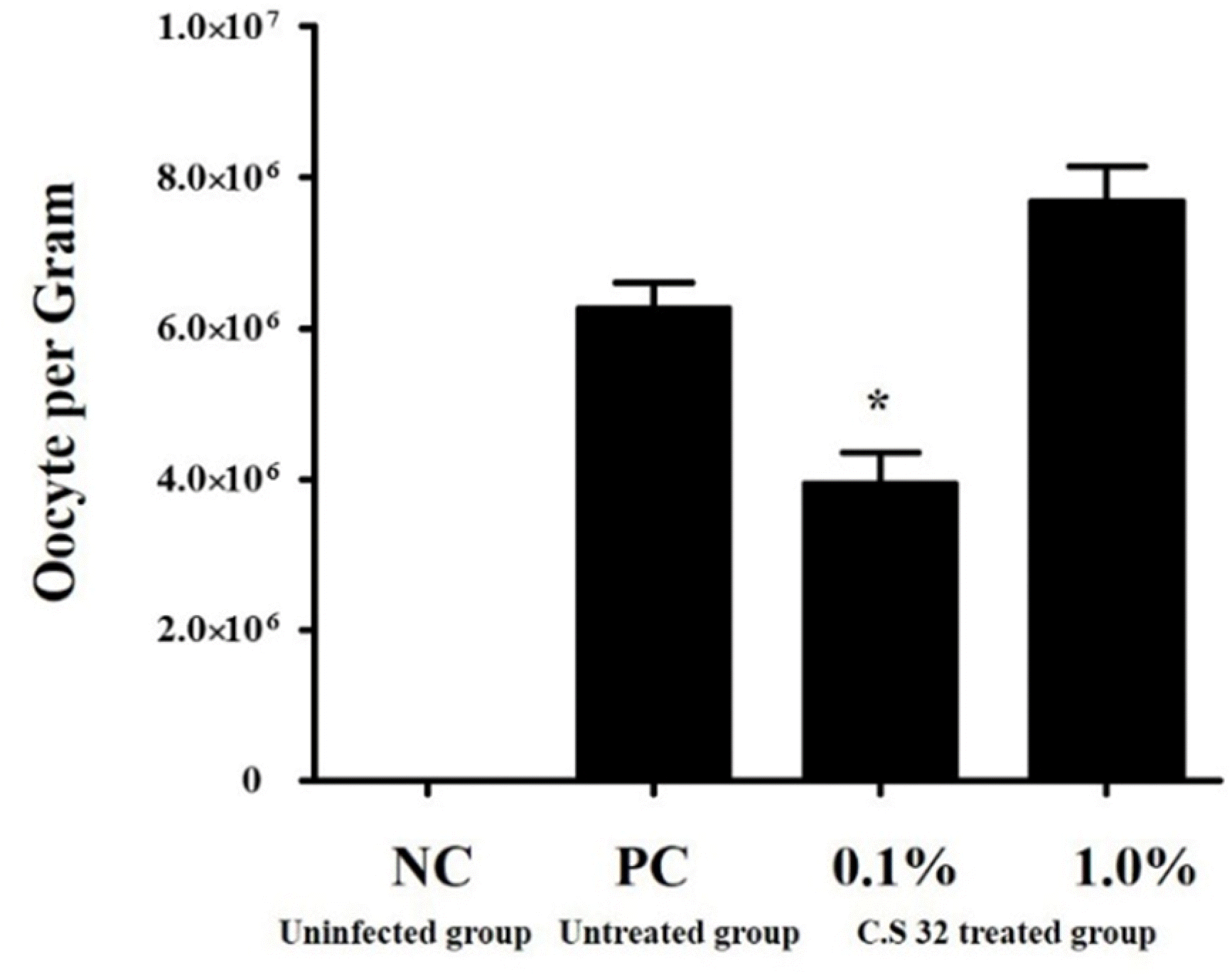 | Fig. 2.Effects of dietary supplementation with CS 32 compounds on oocyte output after challenged with E tenella from days 6 to 10 p.i. NC: negative control (uninfected and untreated group); PC: positive control (infected and untreated group); 0.1%: 0.1% CS 32 compounds group (infected and treated with 0.1% CS 32 compounds); 1.0%: 1.0% CS 32 compounds group (infected and treated with 1.0% CS 32 compounds); n=21 for each group.
∗Significant difference (P<0.05) between PC and 0.1% CS 32 compounds groups.
|
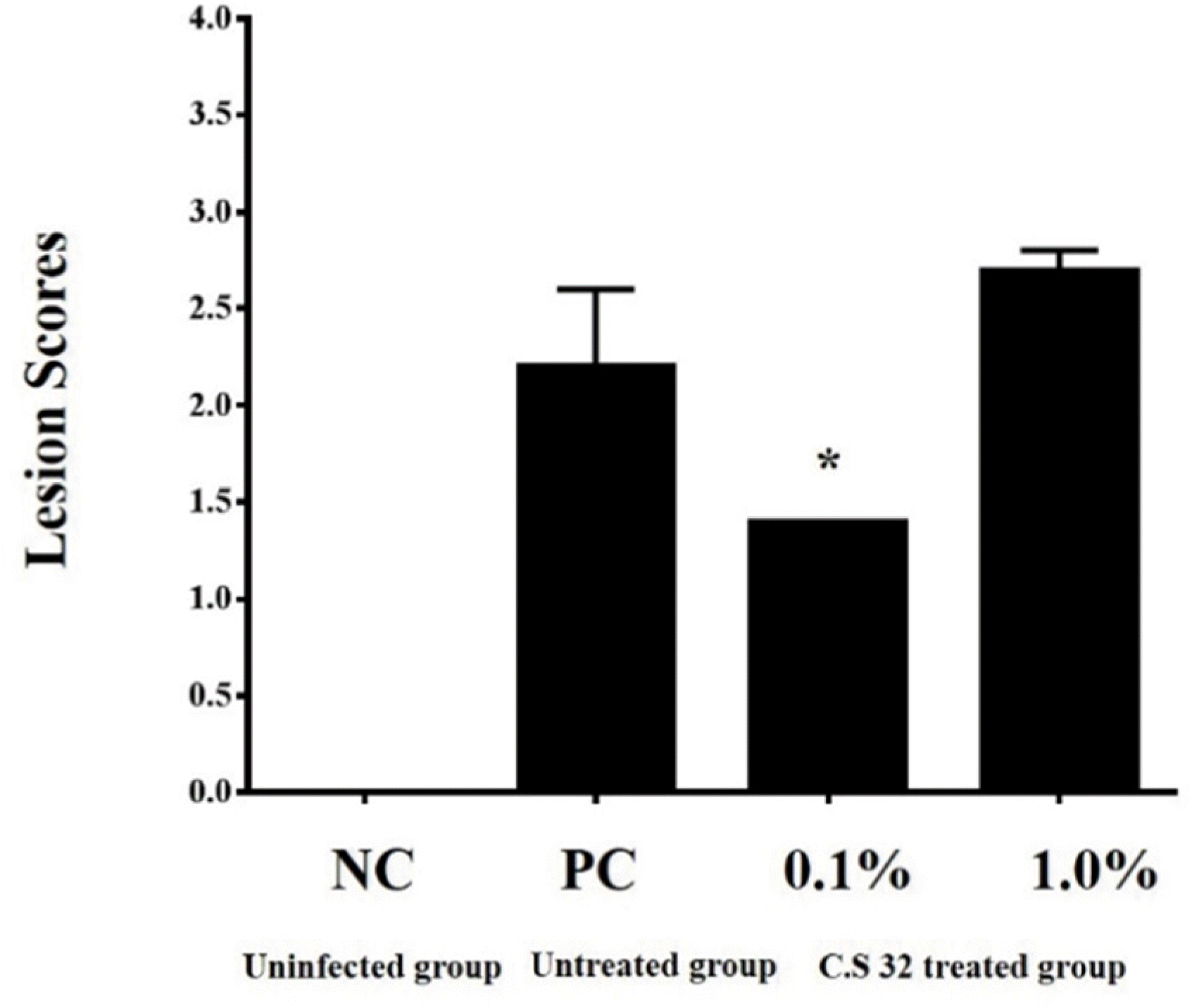 | Fig. 3.Effects of dietary supplementation with CS 32 compounds on lesion score after challenged with E. tenella at 5 days p.i. NC: negative control (uninfected and untreated group); PC: positive control (infected and untreated group); 0.1%: 0.1% CS 32 compounds group (infected and treated with 0.1% CS 32 compounds); 1.0%: 1.0% CS 32 compounds group (infected and treated with 1.0% CS 32 compounds); n=7 for each group.
∗Significant difference (P<0.05) between PC and 0.1% CS 32 compounds groups.
|
Table 1.
Chicken lesion score technique for E. tenella in fection by Johnson and Reid




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download