Abstract
Mayaro virus (MAYV) is a mosquito-transmitted alphavirus that produces an acute, usually non-fatal, febrile illness including Mayaro fever. Like other alphaviruses, the MAYV E1 and E2 envelope glycoproteins are major viral surface antigens that play a key role in host recognition and infection. Here, we report expression and purification methods for recombinant MAYV E1 (rE1) and rE2 using a baculovirus system. Enzyme-linked immunosorbent assays (ELISA) revealed that rE1 and rE2 were antigenic and reacted with human anti–MAYV IgG and IgM. Cross-reactivity was also confirmed with human anti-Chikungunya virus (CHIKV) IgG and IgM. Furthermore, we developed an immunochromatographic strip test (IST) with rE2 to diagnose MAYV infection. Thus, purified rE2 may be valuable tool for rapidly diagnosing MAYV infection.
REFERENCES
1). Muñ oz M, Navarro JC. [Mayaro: a re-emerging Arbovirus in Venezuela and Latin America]. Biomedica. 2012; 32:286–302.
2). Smith JL, Pugh CL, Cisney ED, Keasey SL, Guevara C, Ampuero JS, et al. Human Antibody Responses to Emerging Mayaro Virus and Cocirculating Alphavirus Infections Examined by Using Structural Proteins from Nine New and Old World Lineages. mSphere. 2018; 3.

3). Suhrbier A, Jaffar-Bandjee MC, Gasque P. Arthritogenic alphaviruses–an overview. Nat Rev Rheumatol. 2012; 8:420–9.

4). Choi H, Kudchodkar SB, Reuschel EL, Asija K, Borole P, Ho M, et al. Protective immunity by an engineered DNA vaccine for Mayaro virus. PLoS Negl Trop Dis. 2019; 13:e0007042.

5). Powers AM, Aguilar PV, Chandler LJ, Brault AC, Meakins TA, Watts D, et al. Genetic relationships among Mayaro and Una viruses suggest distinct patterns of transmission. Am J Trop Med Hyg. 2006; 75:461–9.

6). Rodrí guez-Morales AJ, Paniz-Mondolfi AE, Villamil-Gó mez WE, Navarro JC. Mayaro, Oropouche and Venezuelan Equine Encephalitis viruses: Following in the footsteps of Zika? Travel Med Infect Dis. 2017; 15:72–3.
7). Auguste AJ, Liria J, Forrester NL, Giambalvo D, Moncada M, Long KC, et al. Evolutionary and Ecological Characterization of Mayaro Virus Strains Isolated during an Outbreak, Venezuela, 2010. Emerg Infect Dis. 2015; 21:1742–50.

8). Tesh RB, Watts DM, Russell KL, Damodaran C, Calampa C, Cabezas C, et al. Mayaro virus disease: an emerging mosquito-borne zoonosis in tropical South America. Clin Infect Dis. 1999; 28:67–73.

9). Esposito DLA, Fonseca BALD. Will Mayaro virus be responsible for the next outbreak of an arthropod-borne virus in Brazil? Braz J Infect Dis. 2017; 21:540–4.

10). Cheng RH, Kuhn RJ, Olson NH, Rossmann MG, Choi HK, Smith TJ, et al. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell. 1995; 80:621–30.

11). Tello D, Rodrí guez-Rodrí guez M, Yé lamos B, Gó mez-Gutié rrez J, Peterson DL, Gavilanes F. High-yield production of a chimeric glycoprotein based on permuted E1 and E2 HCV envelope ectodomains. J Virol Methods. 2015; 213:38–44.

12). Masoomi Dezfooli S, Tan WS, Tey BT, Ooi CW, Hussain SA. Expression and purification of the matrix protein of Nipah virus in baculovirus insect cell system. Biotechnol Prog. 2016; 32:171–7.

13). Tello D, Rodrí guez-Rodrí guez M, Yé lamos B, Gó mez-Gutié rrez J, Ortega S, Pacheco B, et al. Expression and structural properties of a chimeric protein based on the ectodomains of E1 and E2 hepatitis C virus envelope glycoproteins. Protein Expr Purif. 2010; 71:123–31.

14). Lombana L, Ortega-Atienza S, Gó mez-Gutié rrez J, Yé lamos B, Peterson DL, Gavilanes F. The deletion of residues 268-292 of E1 impairs the ability of HCV envelope proteins to induce pore formation. Virus Res. 2016; 217:63–70.

15). Mackay IM, Arden KE. Mayaro virus: a forest virus primed for a trip to the city? Microbes Infect. 2016; 18:724–34.

16). Pinheiro FP, Freitas RB, Travassos da Rosa JF, Gabbay YB, Mello WA, LeDuc JW. An outbreak of Mayaro virus disease in Belterra, Brazil. I. Clinical and virological findings. Am J Trop Med Hyg. 1981; 30:674–81.
17). Fumagalli MJ, de Souza WM, Romeiro MF, de Souza Costa MC, Slhessarenko RD, Figueiredo LTM. Development of an Enzyme-Linked Immunosorbent Assay To Detect Antibodies Targeting Recombinant Envelope Protein 2 of Mayaro Virus. J Clin Microbiol. 2019; 57.

18). Rodrí guez-Rodrí guez M, Tello D, Yé lamos B, Gó mez-Gutié rrez J, Pacheco B, Ortega S, et al. Structural properties of the ectodomain of hepatitis C virus E2 envelope protein. Virus Res. 2009; 139:91–9.
19). Powers AM, Brault AC, Shirako Y, Strauss EG, Kang W, Strauss JH, et al. Evolutionary relationships and systematics of the alphaviruses. J Virol. 2001; 75:10118–31.

20). Song C, Li J, Liu J, Liu Q. Simple sensitive rapid detection of Escherichia coli O157: H7 in food samples by label-free immunofluorescence strip sensor. Talanta. 2016; 156-157:42–7.
Fig. 1.
MAYV rE1 and rE2 expression using a recombinant baculovirus/insect system, (a) Schematic representation of MAYV genome organization, (b) Schematic of the pAcGP67A-E1 and pAcGP67A-E2 constructs generated. C-terminal His-tags were used to purify the MAYV E1 and E2 proteins, (c) Western blot analysis of media from pAcGP67A-E1- and pAcGP67A-E2-transfected Sf9 cells using anti-His-tag antibodies conjugated to horseradish peroxidase. Uninfected Sf9 cell cultivation media was used as a negative control (Neg. Ctrl.).
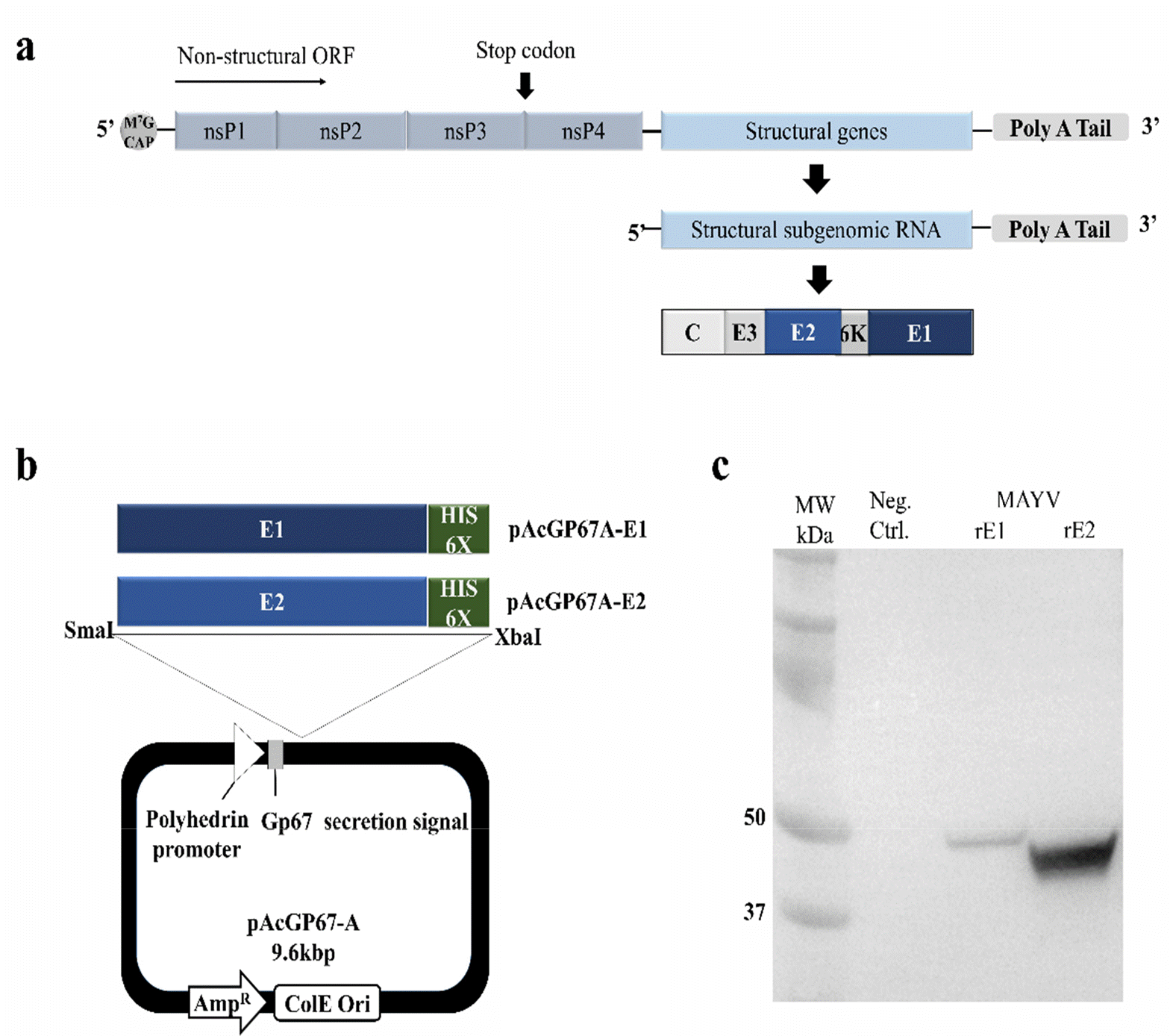
Fig. 2.
Analysis of MAYV rE1 and rE2 overexpression and purification by High Five™ cells, (a) SDS-PAGE of purified MAYV rE1 and rE2. The gel was stained with Coomassie brilliant blue R-250. (b) Western blot analysis of purified MAYV rE1 and rE2. After being transferred to a PVDF membrane, the proteins were detected by anti-His-tag antibodies conjugated to horseradish peroxidase. Uninfected High-Five™ insect cell cultivation media was used as the Neg. Ctrl.
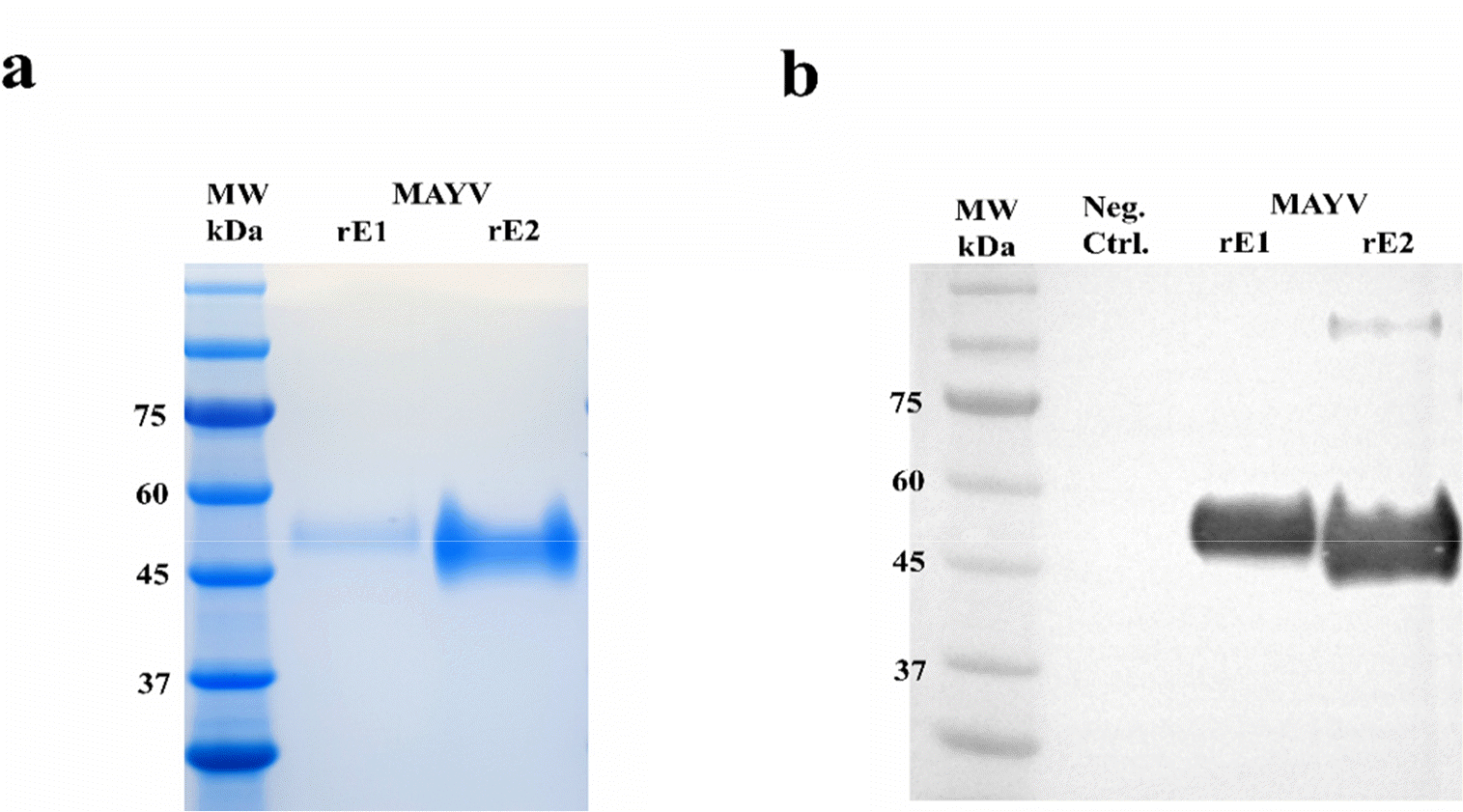
Fig. 3.
rE1 and rE2 reactivity against human anti-MAYV IgG (a) and IgM (b). rE1 and rE2 cross-reactivity were determined by ELISA using anti-MAYV IgG and IgM or anti-CHIKV IgG and IgM. Each microplate well was coated with 100 ng of purified recombinant proteins. Bars indicate the mean absorbance at 450 nm. Error bars indicate standard deviations (SD) of triplicate measurements.
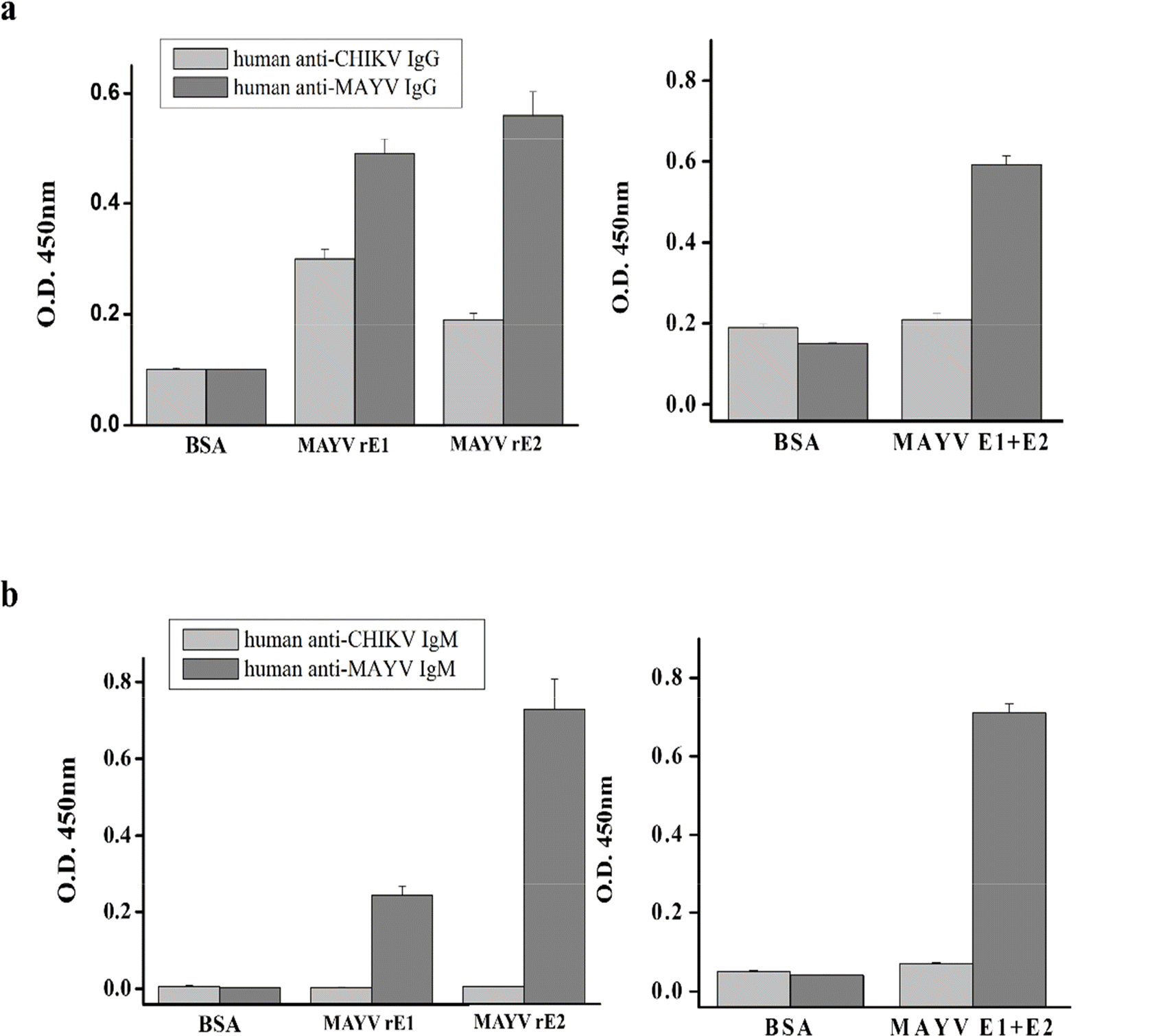
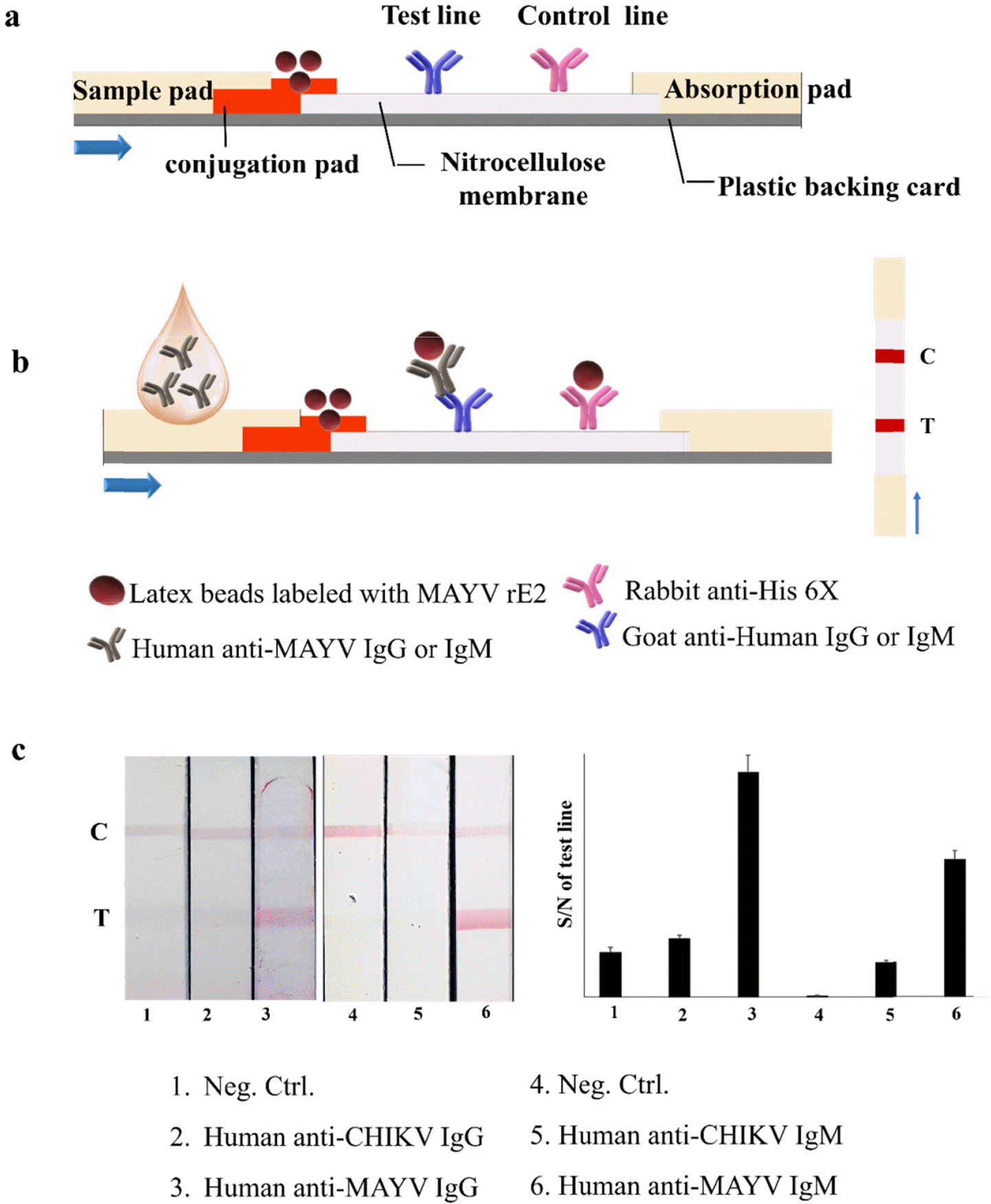
Fig. 4.
Schematic illustration of the MAYV IST assay (a, b) and an image of MAYV IST using rE2 to detect human anti-MAYV IgG and IgM (c). BSA was used as a Neg. Ctrl. Quantification of band intensity at the strip test line. Normalized pixel intensity was quantified using Image software. Intensity values were calculated relative to the control line intensity. The graph presents the ratio of signal to noise (S/N) on the test line.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download