Abstract
Since the identification of succinate's receptor in 2004, studies supporting the involvement of succinate signaling through its receptor in various diseases have accumulated and most of these investigations have highlighted succinate's pro-inflammatory role. Taken with the fact that succinate is an intermediate metabolite in the center of mitochondrial activity, and considering its potential regulation of protein succinylation through succinyl-coenzyme A, a review on the overall multifaceted actions of succinate to discuss whether and how these actions relate to the cellular locations of succinate is much warranted. Mechanistically, it is important to consider the sources of succinate, which include somatic cellular released succinate and those produced by the microbiome, especially the gut microbiota, which is an equivalent, if not greater contributor of succinate levels in the body. Continue learning the critical roles of succinate signaling, known and unknown, in many pathophysiological conditions is important. Furthermore, studies to delineate the regulation of succinate levels and to determine how succinate elicits various types of signaling in a temporal and spatial manner are also required.
Mtabolic citrates such as succinate and NAD+ have recently emerged as important players in various inflammatory conditions. Succinate is an intermediate in the tricarboxylic acid (TCA) cycle and is known to play an essential role in adenosine triphosphate generation in mitochondria. In line with the extensive research focused on the metabolic functions of mitochondria in human pathophysiology, studies that reveal novel functions and regulatory mechanisms of succinate are growing. Here, we review the sources of succinate in the human body and summarize the newly established roles of succinate (which are now expanded beyond its classical metabolic actions) in various human pathophysiological conditions, particularly those that have not been reviewed.
Succinate is a dicarboxylic acid metabolite in the TCA cycle. The accumulation of succinate in mitochondria, the cytosol, and extracellular environment occurs when there is an imbalance between energy demand and oxygen supply. For instance, increased succinate levels have been found during ischemia [1], and exercise [2] due to low oxygen levels. The accumulation of succinate could be a direct consequence of reductions in succinate dehydrogenase (SDH) [3], which catalyzes the conversion of succinate to fumarate in the TCA cycle [45]. In addition to the TCA cycle, other metabolic pathways also contribute to the production of succinate under pathological and physiological conditions. The malate-aspartate shuttle [6] and the purine nucleotide shuttle [7] can increase mitochondrial fumarate, which triggers the reverse activity of SDH, converting fumarate to succinate [8]. The gamma-aminobutyric acid (GABA) shunt also intersects with the TCA cycle via succinate. GABA is synthesized and recycled from α-ketoglutarate that is generated by the TCA cycle into succinate via glutamate, GABA, and succinic semialdehyde. The fact that succinate is involved in multiple metabolic pathways indicates its potential as an intermediate metabolite as well as a signaling transmitter which may quickly transduce metabolic cues.
Succinate is an important metabolite in both host and microbial metabolic processes. While the mitochondria are a physiological source of succinate in ‘sterile’ tissues, the distal gastrointestinal tract is densely populated with microbes that produce succinate as a byproduct of anaerobic fermentation [910111213]. The major producers of succinate in the mammalian gut are bacteria that belong to the Bacteroidetes phylum and the succinate pathway is the dominant route for the generation of propionate, which is found mainly in Bacteroides spp. and Prevotella spp. [9]. Interestingly, the prevalence of Bacteroides and Prevotella represent an intestinal microbiota signature in patients with type 2 diabetes [14]. It is plausible that the altered microbiota contribute to increased levels of succinate levels found in obese and diabetic individuals [15].
Succinate's role as a signaling transducer includes at least three levels of regulation: as an intermediate metabolite to regulate cellular metabolism and respiration, as a substrate for succinyl-coenzyme A (CoA) to conduct protein post-translational modification (PTM) via succinylation, and as a hormone-like molecule to activate succinate receptors.
The role of intracellular succinate as a metabolite which stabilizes hypoxia-inducible factor (HIF)-1α in response to hypoxia [1617], enhances interleukin-1β (IL-1β) production during inflammation in innate immune signaling [18] and stimulates macrophage polarization has been well-characterized [19]. Emerging evidence indicates there is also a regulatory role of succinate in energy expenditure, weight control, and metabolic homeostasis [202122]. Notably, succinate is one of the metabolites that is increased in the plasma in response to exercise and up-regulates the expression of a transcriptional regulator of glucose utilization and lipid metabolism genes in skeletal muscle in vitro [2]. The reversible reaction of succinyl-CoA to succinate is catalyzed by succinyl-CoA synthetase (succinate thiokinase). Succinate can directly influence the level of succinyl-CoA and the lysine succinylation of proteins. Lysine succinylation has recently been identified as a PTM that involves an addition of a succinyl group (-CO-CH2-CH2-CO2H) to a lysine residue in a protein. Lysine succinylation extensively regulates metabolic enzyme activities in mitochondria [2324]. It is interesting that SDH is activated by lysine succinylation [2325] suggesting a self-regulatory mechanism of succinate levels in mitochondria. Extensive lysine succinylation has been found in enzymes involved in mitochondrial metabolism, including the TCA cycle, and fatty acid metabolism [23]. In mammalian cells, it often occurs at the same lysine residues as acetylation [2627]. Notably, succinyl groups contain a negatively charged carboxyl group, indicating succinylation and acetylation are functionally distinct. Indeed, SDH activity is reduced by lysine acetylation [2428] and stimulated by succinylation [23]. The increased lysine acetylation has been found in mesangial and tubular cells exposed to high levels of glucose [29] suggesting that acetylation reduces SDH activity causing succinate accumulation in high glucose environments. Whether and how cells orchestra succinate, succinyl-CoA and succinylation to regulate cell metabolism is an intriguing and yet to be explored topic.
Succinate is exported from mitochondria to the cytosol by the dicarboxylate carrier in exchange for Pi [30]. Tissue or cell type-specific plasma membrane dicarboxylate transporters, likely members of the organic anion transporter families, and sodium-dicarboxylate exchangers are responsible for succinate transport across the plasma membrane. For example, the solute carrier family 26 member 6 (Slc26a6) is an anion-exchanger expressed in the apical membrane of the kidney proximal tubule. Slc26a6-null mice exhibited greater renal and intestinal sodium-dependent succinate uptake than wild-type mice [31]. However, we do not understand what the tissue-specific carriers are and how they are regulated in oxidative or metabolic stress environments to modulate succinate accumulation. As pointed out by Ariza et al. [32], the use of specific antagonists or knockout mice for these cotransporters may reveal pathways involved in pathological states due to succinate accumulation.
Cytosol succinate at elevated levels may be released from cells [3] where it acts as an extracellular mediator that signals via the succinate receptor 1 (SUCNR1) [33]. Succinate/SUCNR1 activation in stress situations and pro-inflammatory actions have been extensively reviewed [323435]. SUCNR1 is a G protein-coupled receptor (GPR91) expressed in various types of cells and tissues (Fig. 1), and its expression is substantially higher in fat tissues, followed by liver, kidney, pancreas, spleen, skin, lymphonodus, gall bladder, thymus and intestine. The function of succinate/SUCNR1 signaling in some of these tissues have been reported. For example, succinate from ischemic hepatocytes triggerd SUCNR1 expressed in quiescent hepatic stellate cells and accelerated stellate cell activation [36]. Succinate activation of SUCNR1 is considered to be a renal and cardiovascular sensory receptor in blood pressure regulation [37]. SUCNR1 activation in the afferent arterioles of the glomerulus increases renin release and induces hypertension [38]. Succinate has been observed to induce a multifaceted type 2 immune response through SUCNR1-expressing tuft cells in the small intestine [3940] which strongly support that gut microbiome derived succinate could be a key mediator of the crosstalk between the microbiome and host immunity.
Of note, SUCNR1 shares a high level of sequence homology with the family of P2Y purinoreceptors [41] and indeed, succinate has been suggested to interfere with the P2Y1 receptor and may mediate calcium mobilization in accumbal astrocytes [42]. Furthermore, the extrasynaptic GABAB receptor sites of rat globus pallidus, a motoric effector area in the nucleus accumbens, has been shown to be succinate-sensitive [43]. Further investigation regarding succinate signaling mediated through SUCNR1 and other potential succinate receptors, especially in the brain, are warranted. It is intriguing to assess the affinity of succinate to different receptors and whether its default receptors vary in different cell types.
The regulatory role of accumulated succinate and its signaling through SUCNR1 has been reported and reviewed in several pathophysiological conditions [89323435364445]. Here, without repeating the well-documented role of succinate signaling through its modulation of the expression of pro-inflammatory cytokines in macrophages, we will highlight its role in a few less-studied areas such as bone, oral health and allergy.
According to the Centers for Disease Control and Prevention the United States, approximately 26.9% (10.9 million) of the population aged 65 years or older have diabetes. Diabetes is associated with a higher risk of sustaining osteoporotic fractures than their non-diabetic counterparts. According to the International Osteoporosis Foundation, osteoporosis affects approximately 10% of women aged 60 and this rate increases increasing steadily to 67% in women aged 90. Aging is a degenerative process caused by accumulated damage that leads to cellular dysfunction, tissue failure, and death [46]. Accumulating evidence suggests a causative link between mitochondrial dysfunction and major phenotypes associated with aging [46]. Consequently, mitochondrial dysfunction could lead to skeletal aging. One major consequence of age-associated mitochondrial dysfunction is oxidative damage caused by an accumulation of reactive oxygen species (ROS). ROS, such as the superoxide radical and H2O2 are crucial components that regulate the differentiation of osteoclasts [4748]. Age-associated mitochondrial dysfunction also leads (unavoidably) to an accumulation of intermediate metabolites [4950515253]. It is known that SDH activity is directly related to life-span. SDH mutations lead to a shortened life span, impaired respiration and overproduction of superoxide in Caenorhabditis elegans [54] and Drosophila [55]. Restoration of SDH-positive mitochondrial density improves metabolic efficiency in aged rats [56]. SDH deficiency could cause an abnormal accumulation of succinate, which could directly contribute to the age-related degeneration of organs expressing SUCNR1. Succinate elevation has indeed been connected to skeletal diseases such as osteoporosis [57], rheumatoid arthritis [58], and osteoarthritis [59]. We have demonstrated the expression of SUCNR1 in bone marrow myeloid lineage cells, including osteoclasts, and succinate elevation induce bone loss through osteoclastogenesis [57]. Succinate is abundant in synovial fluids taken from rheumatoid arthritis patients and exacerbates rheumatoid arthritis through production of IL-1β [58]. Succinate seems to be a “senile-metabolite” tightly related to aging which warrants further study to reveal its role in more aging-related tissue degenerations.
Periodontitis is a common chronic inflammatory disease characterized by the destruction of the supporting structures of the teeth (the periodontal ligament and alveolar bone) [60616263]. It is highly prevalent and has multiple negative impacts on quality of life. Epidemiological data confirm that diabetes is a major risk factor for periodontitis with approximately five-fold higher susceptibility. Over time, diabetes, a widespread chronic disorder, affects oral health. According to the International Diabetes Federation, the number of people with diabetes has risen from 108 million in 1980 to 422 million in 2014, and it is estimated that by 2040 this number will increase to 642 million (http://www.idf.org/). As mentioned previously, succinate elevation is associated with hyperglycemia and thus, succinate signaling is directly implicated in diabetes-related complications. The mechanisms underlying the links between these two conditions are not completely understood, but involve aspects of metabolic dysfunction, immune functioning, cytokine biology, and bacterial colonization. Succinate is elevated in the gingival crevicular fluid of periodontal patients [6465]. The local elevation of succinate levels may lead to SUCNR1 activation in the periodontium, which in turn will increase the expression of inflammatory mediators and bone resorption. Recently, a study demonstrated that periodontal ligament cells exhibited succinate elevation in response to Porphyromonas gingivalis [66], supporting that targeting succinate signaling could be a therapeutic strategy for chronic periodontitis.
Hypersensitivity reactions associated with succinate have been identified in case reports [6768] of immediate allergic reactions to methylprednisolone sodium succinate, but not to methylprednisolone without succinate ester. However, it is still debatable whether succinate itself is immunogenic or if it only increases the immunogenicity of the steroid molecule [6970]. Augmented allergic contact dermatitis was observed in SUCNR1-deficient mice sensitized and challenged with oxazolone; additionally, succinate signaling is required for normal mast cell development [71]. Interestingly, SUCNR1-deficient mice do not have exacerbation of asthma or mast cell-dependent arthritis, suggesting that the absence of succinate signaling does not exacerbate autoimmune pathology [71]. Recently, it was reported that succinate levels are highly upregulated in patients with mild and persistent asthma [72], likely due to hypoxic stress. It is plausible that succinate signaling is involved in both normal and manifested cell functions in allergic reactions. More studies on the role of SUCNR1 activation (or absence) in various cell types will help to delineate succinate signaling in the development of allergic and inflammatory responses.
In cancer cells, high levels of aerobic glycolysis called the “Warburg effect” is the key style of cancer metabolism [73]. SDH, one of a mitochondrial TCA cycle enzyme that converts succinate into fumarate [74], has been demonstrated to be a tumor suppressor [75]. Downregulation of SDH results in a cytosolic accumulation of succinate and generates oncogenic signaling from the mitochondria to the cytosol. Cytosolic succinate inhibits HIF-α prolyl hydroxylase, and activities and protects HIF-1α degradation [76]. This constitutively activated HIF-1α modulates essential genes involved in cancer cell division and positively supports tumor growth [77]. Indeed, downregulation of SDH has been found in human cancers, including stomach and colon [78], and somatic or inherited mutations of SDH lead to pheochromocytoma, paraganglioma, or renal cell carcinoma [79].
Meanwhile, the stabilization of HIF-1α by a cytosolic accumulation of succinate induces IL-1β production, which is a key pro-inflammatory cytokine [18]. In tumor microenvironments, IL-1β can polarize macrophages into the M1 subtype, which can attack cancer cells and play a major role in host defense mechanisms [80]. Furthermore, several lines of evidence indicate succinate directly stimulates immune cells to produce IL-1β and that it contributes to inflammatory conditioning. In the presence of lipopolysaccharides, succinate simultaneously enhances IL-1β production in bone marrow-derived dendritic cells [81]. Succinate also enhances the antigen presenting potentials of dendritic cells, which enables an adaptive response and exacerbates inflammation [82]. A recent study by Wu et al. [83] showed that breast cancer cells release succinate into their microenvironment and activate SUCNR1 signaling to polarize macrophages into tumor-associated macrophages, which promotes cancer cell migration, invasion and even metastasis. Succinate may also aggravate the progression toward nonalcoholic fatty liver disease at the onset of obesity through enhanced protein stabilization and activity [84].
Collectively, succinate has dual conflicting actions in cancer microenvironments. It can positively stimulate cancer cell survival directly or indirectly through manipulating tumor micro-environment to favor its progression. In contrast, succinate signaling may also contribute to anti-tumor immunities by activating host defensive immune cells such as M1 macrophages or dendritic cells. Further studies are needed to understand these finely tuned mechanisms.
It is plausible that the three levels of succinate regulatory mechanisms occur in a highly orchestrated temporal manner at specific cellular organelles. Initially, under normal physiological conditions, succinate primarily acts as an intermediate metabolite to regulate cellular metabolism and respiration. Through reversible conversion to succinyl-CoA, succinate may quickly respond to metabolic cues inside mitochondria and the cytosol to regulate the target proteins' activity by succinylation. Eventually, the release of excess succinate extracellularly enables its action as a ligand to activate SUCNR1 and initiate pro-inflammatory responses. Future studies focused on delineating the timing and regulation of succinate release in the context of various pathophysiological conditions and tissues will be helpful to reveal the complexity of succinate's multifaceted role.
Figures and Tables
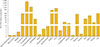
Fig. 1
Expression levels of signals via the succinate receptor 1 (SUCNR1) mRNA in various tissues of mice. A 12-week-old C57BL/6J male mouse was euthanized before the organs were isolated and snap frozen with liquid nitrogen. The samples were stored at −80℃ until RNA extraction. An age matched C57BL/6J female mouse's ovary was collected and stored in the same way. Total RNA was isolated using TRI Reagent (Sigma-Aldrich), and purified using the RNeasy kit (Qiagen). Following TaqMan reverse transcription, polymerase chain reaction was performed in a BioRad 384-module using a SYBR Green (Applied Biosystems) method with primers for the indicated genes: β-actin forward primer: 5′-AGCCATGTACGTAGCCATCC-3′; β-actin reverse primer:5′-CTCTCAGCTGTGGTGGTGAA-3′. SUCNR1 forward primer: 5′-GACAGAAGCCGACAGCAGAAT-3′; SUCNR1 reverse primer: 5′-TAGACATTGCTGCTGTTCCAGTT-3′.
ACKNOWLEDGMENTS
This study was funded by NIH grants AG055787 (Xin Li), DE027074 (Xin Li, Deepak Saxena), the NYU Provost Mega-grants Initiative award (Xin Li) and the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education NRF-2017R1A2B4005563 (Sun Wook Cho).
References
1. Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014; 515:431–435.

2. Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010; 2:33ra37.

4. Cecchini G. Function and structure of complex II of the respiratory chain. Annu Rev Biochem. 2003; 72:77–109.

5. Ackrell BA. Progress in understanding structure-function relationships in respiratory chain complex II. FEBS Lett. 2000; 466:1–5.

6. Lane M, Gardner DK. Mitochondrial malate-aspartate shuttle regulates mouse embryo nutrient consumption. J Biol Chem. 2005; 280:18361–18367.

7. Lowenstein JM. Ammonia production in muscle and other tissues: the purine nucleotide cycle. Physiol Rev. 1972; 52:382–414.

8. Haas R, Cucchi D, Smith J, Pucino V, Macdougall CE, Mauro C. Intermediates of metabolism: from bystanders to signalling molecules. Trends Biochem Sci. 2016; 41:460–471.

9. Connors J, Dawe N, Van Limbergen J. The role of succinate in the regulation of intestinal inflammation. Nutrients. 2019; 11:25.

10. Ferreyra JA, Wu KJ, Hryckowian AJ, Bouley DM, Weimer BC, Sonnenburg JL. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe. 2014; 16:770–777.
11. Gao YD, Zhao Y, Huang J. Metabolic modeling of common Escherichia coli strains in human gut microbiome. Biomed Res Int. 2014; 2014:694967.
12. Li M, Gu D, Xu N, Lei F, Du L, Zhang Y, et al. Gut carbohydrate metabolism instead of fat metabolism regulated by gut microbes mediates high-fat diet-induced obesity. Benef Microbes. 2014; 5:335–344.

13. Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003; 62:67–72.

14. Leite AZ, Rodrigues NC, Gonzaga MI, Paiolo JCC, de Souza CA, Stefanutto NAV, et al. Detection of increased plasma interleukin-6 levels and prevalence of Prevotella copri and Bacteroides vulgatus in the feces of type 2 diabetes patients. Front Immunol. 2017; 8:1107.

15. Serena C, Ceperuelo-Mallafre V, Keiran N, Queipo-Ortuno MI, Bernal R, Gomez-Huelgas R, et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018; 12:1642–1657.

16. Agani FH, Pichiule P, Chavez JC, LaManna JC. The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J Biol Chem. 2000; 275:35863–35867.

17. Piantadosi CA, Suliman HB. Transcriptional regulation of SDHa flavoprotein by nuclear respiratory factor-1 prevents pseudo-hypoxia in aerobic cardiac cells. J Biol Chem. 2008; 283:10967–10977.

18. Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013; 496:238–242.

19. Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016; 167:457–470.

20. Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018; 560:102–106.

21. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000; 404:661–671.

22. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006; 443:289–295.

23. Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013; 50:919–930.

24. Lin H, Su X, He B. Protein lysine acylation and cysteine succination by intermediates of energy metabolism. ACS Chem Biol. 2012; 7:947–960.

25. Gibson GE, Xu H, Chen HL, Chen W, Denton TT, Zhang S. Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines. J Neurochem. 2015; 134:86–96.

26. Weinert BT, Scholz C, Wagner SA, Iesmantavicius V, Su D, Daniel JA, et al. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013; 4:842–851.

27. Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011; 7:58–63.

28. Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010; 49:304–311.

29. Kosanam H, Thai K, Zhang Y, Advani A, Connelly KA, Diamandis EP, et al. Diabetes induces lysine acetylation of intermediary metabolism enzymes in the kidney. Diabetes. 2014; 63:2432–2439.

30. Palmieri F, Prezioso G, Quagliariello E, Klingenberg M. Kinetic study of the dicarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1971; 22:66–74.

31. Ohana E, Shcheynikov N, Moe OW, Muallem S. SLC26A6 and NaDC-1 transporters interact to regulate oxalate and citrate homeostasis. J Am Soc Nephrol. 2013; 24:1617–1626.

32. Ariza AC, Deen PM, Robben JH. The succinate receptor as a novel therapeutic target for oxidative and metabolic stress-related conditions. Front Endocrinol (Lausanne). 2012; 3:22.

33. He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004; 429:188–193.

34. Mills E, O'Neill LA. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 2014; 24:313–320.

35. Murphy MP, O'Neill LAJ. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell. 2018; 174:780–784.

36. Correa PR, Kruglov EA, Thompson M, Leite MF, Dranoff JA, Nathanson MH. Succinate is a paracrine signal for liver damage. J Hepatol. 2007; 47:262–269.

37. Pluznick JL. Renal and cardiovascular sensory receptors and blood pressure regulation. Am J Physiol Renal Physiol. 2013; 305:F439–F444.

38. Robben JH, Fenton RA, Vargas SL, Schweer H, Peti-Peterdi J, Deen PM, et al. Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int. 2009; 76:1258–1267.

39. Lei W, Ren W, Ohmoto M, Urban JF Jr, Matsumoto I, Margolskee RF, et al. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc Natl Acad Sci U S A. 2018; 115:5552–5557.

40. Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, et al. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity. 2018; 49:33–41.

41. Wittenberger T, Schaller HC, Hellebrand S. An expressed sequence tag (EST) data mining strategy succeeding in the discovery of new G-protein coupled receptors. J Mol Biol. 2001; 307:799–813.
42. Molnar T, Dobolyi A, Nyitrai G, Barabas P, Heja L, Emri Z, et al. Calcium signals in the nucleus accumbens: activation of astrocytes by ATP and succinate. BMC Neurosci. 2011; 12:96.
43. Molnar T, Fekete EK, Kardos J, Simon-Trompler E, Palkovits M, Emri Z. Metabolic GHB precursor succinate binds to gamma-hydroxybutyrate receptors: characterization of human basal ganglia areas nucleus accumbens and globus pallidus. J Neurosci Res. 2006; 84:27–36.
44. de Castro Fonseca M, Aguiar CJ, da Rocha Franco JA, Gingold RN, Leite MF. GPR91: expanding the frontiers of Krebs cycle intermediates. Cell Commun Signal. 2016; 14:3.

47. Ushio-Fukai M, Rehman J. Redox and metabolic regulation of stem/progenitor cells and their niche. Antioxid Redox Signal. 2014; 21:1587–1590.

48. Perales-Clemente E, Folmes CD, Terzic A. Metabolic regulation of redox status in stem cells. Antioxid Redox Signal. 2014; 21:1648–1659.

49. Levtchenko E, de Graaf-Hess A, Wilmer M, van den Heuvel L, Monnens L, Blom H. Altered status of glutathione and its metabolites in cystinotic cells. Nephrol Dial Transplant. 2005; 20:1828–1832.

50. Seidman MD, Bai U, Khan MJ, Quirk WS. Mitochondrial DNA deletions associated with aging and presbyacusis. Arch Otolaryngol Head Neck Surg. 1997; 123:1039–1045.

51. Orsucci D, Filosto M, Siciliano G, Mancuso M. Electron transfer mediators and other metabolites and cofactors in the treatment of mitochondrial dysfunction. Nutr Rev. 2009; 67:427–438.

52. Abdul-Ghani MA, Muller FL, Liu Y, Chavez AO, Balas B, Zuo P, et al. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab. 2008; 295:E678–E685.

53. Lange LG, Sobel BE. Mitochondrial dysfunction induced by fatty acid ethyl esters, myocardial metabolites of ethanol. J Clin Invest. 1983; 72:724–731.

54. Huang J, Lemire BD. Mutations in the C. elegans succinate dehydrogenase iron-sulfur subunit promote superoxide generation and premature aging. J Mol Biol. 2009; 387:559–569.

55. Walker DW, Hajek P, Muffat J, Knoepfle D, Cornelison S, Attardi G, et al. Hypersensitivity to oxygen and shortened lifespan in a Drosophila mitochondrial complex II mutant. Proc Natl Acad Sci U S A. 2006; 103:16382–16387.

56. Balietti M, Giorgetti B, Di Stefano G, Casoli T, Platano D, Solazzi M, et al. A ketogenic diet increases succinic dehydrogenase (SDH) activity and recovers age-related decrease in numeric density of SDH-positive mitochondria in cerebellar Purkinje cells of late-adult rats. Micron. 2010; 41:143–148.

57. Guo Y, Xie C, Li X, Yang J, Yu T, Zhang R, et al. Succinate and its G-protein-coupled receptor stimulates osteoclastogenesis. Nat Commun. 2017; 8:15621.

58. Littlewood-Evans A, Sarret S, Apfel V, Loesle P, Dawson J, Zhang J, et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016; 213:1655–1662.
59. Shen J, Wang C, Ying J, Xu T, McAlinden A, O'Keefe RJ. Inhibition of 4-aminobutyrate aminotransferase protects against injury-induced osteoarthritis in mice. JCI Insight. 2019; 4:128568.

60. Al-Mashat HA, Kandru S, Liu R, Behl Y, Desta T, Graves DT. Diabetes enhances mRNA levels of proapoptotic genes and caspase activity, which contribute to impaired healing. Diabetes. 2006; 55:487–495.

61. Graves DT, Al-Mashat H, Liu R. Evidence that diabetes mellitus aggravates periodontal diseases and modifies the response to an oral pathogen in animal models. Compend Contin Educ Dent. 2004; 25:38–45.
62. Pacios S, Kang J, Galicia J, Gluck K, Patel H, Ovaydi-Mandel A, et al. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 2012; 26:1423–1430.

63. Wu YY, Xiao E, Graves DT. Diabetes mellitus related bone metabolism and periodontal disease. Int J Oral Sci. 2015; 7:63–72.

64. Lu RF, Feng XH, Xu L, Meng HX. Clinical and putative periodontal pathogens' features of different sites with probing depth reduction after non-surgical periodontal treatment of patients with aggressive periodontitis. Beijing Da Xue Xue Bao Yi Xue Ban. 2015; 47:13–18.
65. Ohwaki K. High carboxylic acid level in the gingival crevicular fluid (GCF) of the patients with advanced periodontal disease. Nihon Shishubyo Gakkai Kaishi. 1988; 30:985–995.
66. Su W, Shi J, Zhao Y, Yan F, Lei L, Li H. Porphyromonas gingivalis triggers inflammatory responses in periodontal ligament cells by succinate-succinate dehydrogenase-HIF-1α axis. Biochem Biophys Res Commun. 2020; 522:184–190.

67. Caimmi S, Caimmi D, Bousquet PJ, Demoly P. Succinate as opposed to glucocorticoid itself allergy. Allergy. 2008; 63:1641–1643.

68. Holz W, Ludwig A, Forst H. Anaphylactic shock following intravenous hydrocortisone succinate administration. Anaesthesist. 2002; 51:187–190.
69. Calogiuri G, Foti C, Nettis E, Di Leo E, Macchia L, Vacca A. Should succinate esters be considered excipients in systemic corticosteroid allergy. J Allergy Clin Immunol Pract. 2019; 7:342–343.

70. Li PH, Wagner A, Thomas I, Watts TJ, Rutkowski R, Rutkowski K. Steroid allergy: clinical features and the importance of excipient testing in a diagnostic algorithm. J Allergy Clin Immunol Pract. 2018; 6:1655–1661.

71. Rubic-Schneider T, Carballido-Perrig N, Regairaz C, Raad L, Jost S, Rauld C, et al. GPR91 deficiency exacerbates allergic contact dermatitis while reducing arthritic disease in mice. Allergy. 2017; 72:444–452.

72. Chang C, Guo ZG, He B, Yao WZ. Metabolic alterations in the sera of Chinese patients with mild persistent asthma: a GC-MS-based metabolomics analysis. Acta Pharmacol Sin. 2015; 36:1356–1366.

74. King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006; 25:4675–4682.

76. Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005; 7:77–85.
77. Masson N, Ratcliffe PJ. Hypoxia signaling pathways in cancer metabolism: the importance of co-selecting interconnected physiological pathways. Cancer Metab. 2014; 2:3.

78. Habano W, Sugai T, Nakamura S, Uesugi N, Higuchi T, Terashima M, et al. Reduced expression and loss of heterozygosity of the SDHD gene in colorectal and gastric cancer. Oncol Rep. 2003; 10:1375–1380.

79. Baysal BE. On the association of succinate dehydrogenase mutations with hereditary paraganglioma. Trends Endocrinol Metab. 2003; 14:453–459.

80. O'Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016; 213:15–23.
81. Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido-Perrig N, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol. 2008; 9:1261–1269.

82. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012; 12:265–277.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download