INTRODUCTION
Reflex and spontaneous movements are not uncommon during the process of determining brain death. The frequency of such movements ranges from 19.2%–75% among all brain dead cases but the reported frequencies widely vary from one study to another.
123456 Recently, organ donations from brain dead patients have become common and thus knowledge about such movements is necessary for accurate diagnoses of brain death. Sufficient evidence on how many brain dead patients exhibit reflex or spontaneous movements and whether there are differences in the incidence of these movements according to cause of brain death will aid physicians in correctly diagnosing brain death. Although many studies have investigated the body movements of patients with suspected brain death, few have applied verified tools and neurological tests as a standard protocol for diagnosis.
345 In Korea, only a single prospective study of 26 adult brain dead patients at a single tertiary medical center was published in 2006.
5 Further studies will enhance the current awareness of these movements and might prevent delays and misinterpretations associated with brain death diagnoses. Therefore, the present study assessed 436 brain dead patients in Korea to determine the frequency, characteristics, and factors associated with the presence of reflex and spontaneous movements.
Go to :

DISCUSSION
Reflex and spontaneous movements associated with brain death have been evaluated in more than 130 studies of various designs ranging from animal studies to cohort studies.
10 However, only a few have employed verified neurological examination and laboratory tests as a standard protocol when diagnosing brain death.
3456 The frequencies of reflex and spontaneous movements differ widely among studies and the most commonly reported movements vary as well. In Korea, a single prospective study of 26 adult patients at a single tertiary medical center in 2006 has been published.
5 To the best of our knowledge, the present study included the largest sample of cases that has been used to investigate movements in brain dead patients.
We defined movements induced by stimulation as reflex movements, whereas self-appearing movements in the absence of stimulation were defined as spontaneous movements. These terms are clinically useful because all movements associated with brain death are divided into these two categories.
9 Except for anecdotal reports, it was possible to confirm the frequency results of two initial retrospective studies published in 1973 that investigated these movements.
12 Ivan
1 reported that reflex or spontaneous movements were present in 39 of 54 brain dead patients (72.2%) and Jorgensen
2 observed these movements in 50 of 63 such patients (79.4%). Both studies found the frequency of such movements to be more than 70%, which is higher than the rates reported by recent studies. These two initial studies had some limitations in that the enrolled patients had not been diagnosed according to the brain death criteria of the American Academy of Neurology (AAN) and the protocols that elicited these movements were not standardized. Subsequently, Dösemeci et al.
3 and Saposnik et al.
4 reported that reflex or spontaneous movements were present in 18 of 134 (13.4%) and 47 of 107 brain dead patients (43.9%), respectively. These studies were prospective, met the AAN criteria for brain death, and performed standardized protocols to elicit movements. Most recently, Hosseini et al.
6 reported reflex or spontaneous movements in 40 of 122 (32.8%) brain dead patients in a 1-year cross-sectional study conducted in a single organ procurement unit. The frequency of movements observed in these studies ranged from 13.4% to 43.9%, which is highly variable. In 2006, a total of 26 adult brain dead patients at a single Korean tertiary medical center were enrolled in a study.
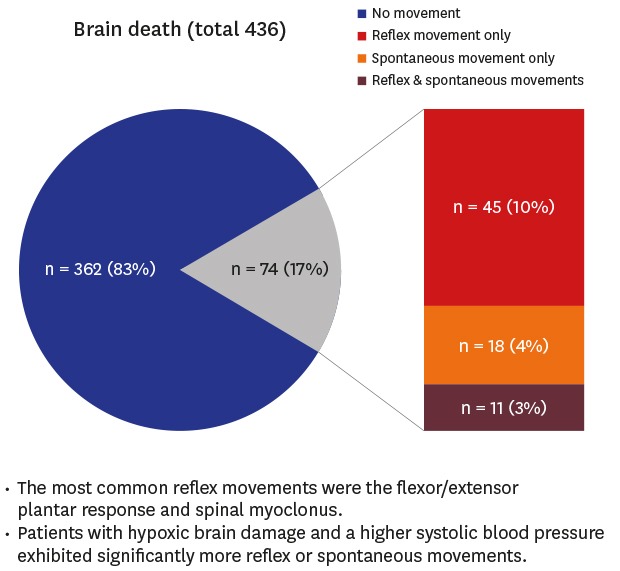
5 Five (19.2%) exhibited reflex or spontaneous body movements during the process of determining brain death. Our results are in line with their reported frequency.
The most frequent movements or common movements are mentioned as important outcomes in previous reports but differ from study to study.
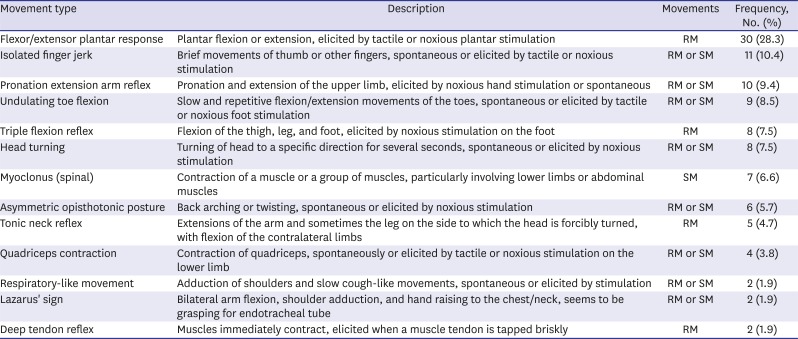
123456 In the present study, the most common movement was flexor/extensor plantar response (
Table 4), which is consistent with the findings of Hosseini et al.
6 Eleven (14.9%) patients exhibited both reflex and spontaneous movements and 25 (33.8%) patients exhibited more than two movement patterns in each patient. The greatest number of accompanied movement patterns are 4. Also, 29 (39.2%) patients exhibited these movements on multiple body areas. These results suggest that reflex and spontaneous movements can appear together in a single patient, and either a single or various movement patterns can occur on several body parts. Han et al.
5 conducted the only previous study on this topic in Korea. They observed these movements in 19.2% of their patients, a similar rate to our findings (17.0%). However, the most common movement and distribution of brain death etiology differed between their study and ours. Han et al.
5 reported that the most common movement was a pronation-extension reflex. In addition, there were no identified factors that were associated with these movements.
In 2005, Saposnik et al.
4 reported that reflex and spontaneous movements were significantly more common when the cause of brain death was focal lesions and that brain dead patients had a high average BP. In addition, Hosseini et al.
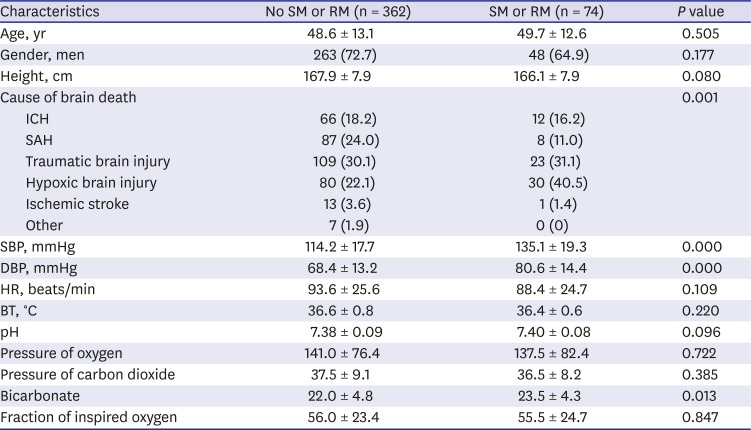
6 reported that the mean SBP was significantly higher in a group of brain dead patients that exhibited movement. We found that brain death was more frequent in patients with hypoxic brain damage than in those who sustained focal lesions, contrary to the findings of Saposnik et al.
4 That study included 107 brain dead patients but only 9 had suffered from hypoxic brain injury and thus it was likely difficult to perform statistical analyses. In contrast to Saposnik et al.,
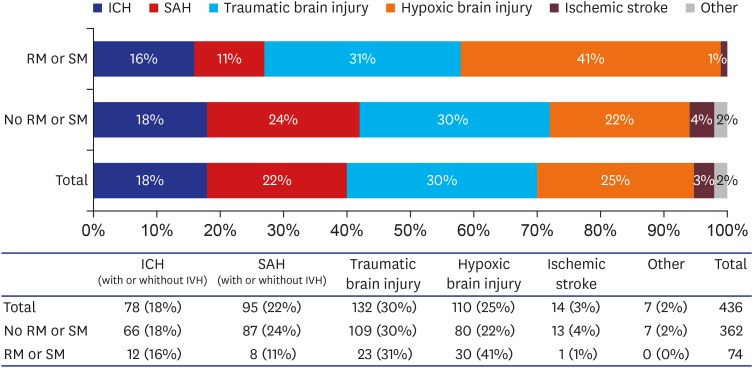
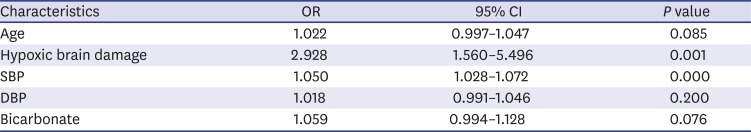
4 we included 436 brain dead patients and 110 (25.2%) had hypoxic brain injury. Of these patients, 80 of 362 (22.0%) patients who did not exhibit movement and 30 of 74 (40.5%) who did exhibit movement had hypoxic brain injury. Although multivariate analyses revealed significant differences between these groups (OR, 2.928; 95% CI, 1.560–5.496;
P = 0.001) (
Table 3), it would be difficult to explain why patients with hypoxic brain injury were more likely to display movements than those with focal brain injuries. Likewise, Saposnik et al.
4 could not provide an explanation regarding why there were more movements in brain dead patients with a focal injury.
A previous study suggested that different types of myoclonus can be observed in the posthypoxic state.
11 We examined whether the significantly higher occurrence of these movements in hypoxic brain injury was associated with posthypoxic myoclonus. Of the 436 brain-dead patients, 110 had hypoxic brain injury. Of these, 30 had reflex or spontaneous movements. We examined the detailed reflex or spontaneous movements of the hypoxic brain injury patients. Myoclonus was observed only in three patients (two spinal myoclonus, one abdominal reflex). The most common movement found was flexor/extensor plantar response, not myoclonus, followed by a triple flexion reflex. The proportion of patients who had myoclonus was relatively small among hypoxic brain injury patients compared with other etiologies of brain death. In addition, myoclonus was observed in 8 of 74 patients with reflex or spontaneous movements and myoclonus appeared more often in patients with traumatic brain injury than in those with hypoxic brain injury (
Fig. 3).
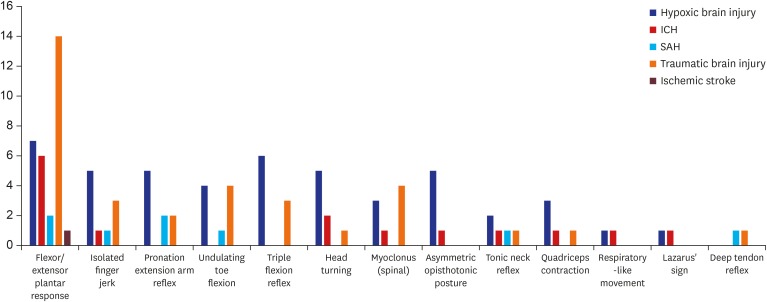 | Fig. 3
Characteristics and frequencies of movements in patients classified by etiology of brain death.
ICH = intracerebral hemorrhage, SAH = subarachnoid hemorrhage.

|
In conclusion, we analyzed reflex and spontaneous movements using a large sample of brain dead patients in Korea and found that 17.0% of patients exhibited these movements during the process of brain death diagnosis; the observed frequency is lower than that reported by previous studies. The occurrence of reflex and spontaneous movements in brain dead patients is not rare but previous studies of this phenomenon that employed relatively similar designs showed different results. In the present study, the flexor/extensor plantar response and isolated finger jerks were the most common movements and more movements were observed in patients with hypoxic brain injury and a higher SBP. If medical staff have sufficient knowledge of the frequency and patterns of these movements in patients with impending brain death, they may be better able to reduce delays in the diagnosis of brain death.
Go to :





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download