Abstract
Objective
Early onset neonatal hypocalcemia (ENH) occurs within 72 hours after birth and screening is recommended for high-risk infants. This study aimed to analyze the risk factors predictive of ENH and evaluate the effectiveness of intervention for ENH in moderate-to-late preterm infants.
Methods
This was a retrospective study, examining moderate-to-late preterm infants at 32–36 weeks of gestational age. Perinatal factors were compared between infants with and without hypocalcemia. Related factors were further analyzed to evaluate the validity of the predictive factors for ENH. The effect of intervention was analyzed by comparing changes in serum calcium levels on day 3 and day 7.
Results
A total of 112 infants were enrolled. There were 65 infants in the non-hypocalcemic group and 47 in the hypocalcemic group. Between the two groups, only lower magnesium levels on day 1 were related to ENH with an odds ratio of 0.247 using a cutoff value of 1.7 mmol/L for hypocalcemia (sensitivity 42.6%, specificity 80.0%, P=0.005). No infant with hypocalcemia exhibited clinical seizures. Intervention was performed with oral calcium supplements or low-phosphorus milk feeding in 30 infants. The intervention was more effective in resolving hypocalcemia than no intervention (n=17).
Conclusion
Lower magnesium levels on day 1 were related to lower calcium levels and a 4-fold higher risk for hypocalcemia. Moderate-to-late preterm infants with hypomagnesemia should be closely monitored for ENH. Additionally, interventions were found to be effective in restoring calcium levels in ENH.
Early onset neonatal hypocalcemia (ENH) often occurs after birth, is lowest at day 3, and recovers spontaneously.1 The incidence of ENH is approximately 40% and is especially common in preterm infants.2 During fetal mineral homeostasis, the placenta actively transports minerals, rather than the intestines and kidneys.34 It results in higher concentrations of calcium, ionized calcium, and phosphate in fetal blood to ensure adequate mineral supply for bone development,4 which suppress fetal parathyroid (PTH) hormones secretion.3 However, when the umbilical cord is cut, placental mineral infusion stops. Due to the lag required for the neonatal PTH, intestines, and kidneys to become fully responsive, ENH is often seen in infants followed by an increase in serum calcium over the following days.4
Even though ENH is known for its spontaneous recovery after an adaptation period, screening is recommended for high-risk infants so that early intervention for ENH1 can prevent events such as hypocalcemic seizures. High-risk infants include babies of extremely low birth weight,5 those born by cesarean section,6 babies fed milk with too much phosphate,7 newborns small for their gestational age,18 and babies who experienced an event of asphyxia.1910 Maternal hypoparathyroidism,111 gestational diabetes mellitus,11213 history of oligohydramnios,14 or preeclampsia1 are also known risk factors for ENH.
Since seizures in hypocalcemic neonates are quite common, prevention is important.15 Even though prophylactic calcium supplementation is recommended in extremely preterm infants,9 there is no consensus regarding the appropriate prophylactic intervention for moderate-to-late preterm infants. Thus, this study aimed to evaluate the risk factors predictive of ENH and the effectiveness of intervention on ENH in moderate-to-late preterm infants.
This was a retrospective study examining moderate-to-late preterm infants who were admitted to the neonatal intensive care units in Korea University Anam and Ansan Hospitals from January 2017 to May 2018. We reviewed the medical charts of preterm babies who were born at gestational ages between 32 weeks and 36 weeks. Babies with lack of laboratory data or maternal history were excluded from the study. Babies who needed parenteral nutrition were also excluded since they were already supplemented with additional calcium. Babies who were discharged within 7 days after birth were excluded, as well.
The hypocalcemic group was defined as babies with serum ionized calcium levels less than 4.0 mg/dL on day 3 of life.111 Perinatal and maternal factors were compared between the hypocalcemic group and the non-hypocalcemic group. They included gestational age, birth weight, cesarean section, Apgar scores, small for gestational age, hypoglycemic status, formula feeding, feeding amount, time to first feeding, maternal hypothyroidism, oligohydramnios, maternal diabetes mellitus, maternal pregnancy-induced hypertension, and maternal magnesium administration before delivery. Infants' ionized calcium, total serum calcium, phosphorous, magnesium, creatinine, and alkaline phosphatase (ALP) levels were compared.111617 Relevant factors were further analyzed to determine whether they had significant predictive value for ENH.
The effect of intervention to improve ENH was analyzed by dividing the hypocalcemic group into the intervention group and the non-intervention group. Oral calcium supplements or low-phosphorous milk feeding were used as interventions.1819 The characteristics of the two groups were compared and changes in laboratory findings from day 3 to day 7 were analyzed to evaluate the effectiveness of the interventions.
In this study, we used maternal cord blood samples on day 1 after birth and peripheral blood on day 3 and day 7 after birth. Total serum calcium, phosphorous, magnesium, creatinine, and ALP levels were measured in laboratory using AU 5800 (Beckman Coulter, Brea, CA, USA) in Anam Hospital, Cobas 8000 (Roche Diagnostic System, Basel, Switzerland) in Ansan Hospital. Infants' serum ionized calcium were measured bedside using a commercial kit (iSTAT EG7®; Abbott, Abbott Park, IL, USA) in both hospitals.
IBM SPSS Statistics ver. 20.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analyses of the collected hospital data. Comparison of categorical variables for the baseline antropometric and demographic data between the two groups was performed by the Chi-square test or Fisher's exact test. Student's t-test or the Mann-Whitney test were used for continuous variables. Logistic regression was used to evaluate the relationship of the predictive factors for ENH. P-values below 0.05 were considered statistically significant.
A total of 211 infants were enrolled and 99 were excluded due to lack of laboratory findings, deficient maternal history, history of parenteral nutrition use, or early discharge within 7 days after birth. The infants were divided into two groups according to their calcium levels on day 3. There were 65 newborns in the non-hypocalcemic group and 47 in the hypocalcemic group. Intervention was performed for 30 infants in the hypocalcemic group, 6 were fed low-phosphorous milk and 24 were given oral calcium. Although it was a retrospective study and the intervention was not performed by precise criteria, most interventions were applied according to the followings because both hospitals shared the same protocol about intervention of ENH; oral calcium was supplied to infants when their ionized calcium level on day 3 was less than 3.5 mg/dL, and low phosphorous milk was fed when the calcium/phosphorous ratio was less than 1.0.
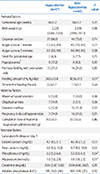
There were significant difference in ionized calcium level on day 3 between hypocalcemic group and non-hypocalcemic group (3.70 [3.55, 3.85] vs. 4.30 mg/dL [3.85, 4.75], P<0.001). Phosphorus (6.0 vs. 5.5 mg/dL, P=0.04) and magnesium (1.8 vs. 1.9 mg/dL, P=0.01) levels on day 1 were significantly different between the hypocalcemic and the non-hypocalcemic group. Feeding amount was significantly different between the hypocalcemic group and the non-hypocalcemic group (34.5 vs. 42.0 mL/kg/day, P=0.01). Other factors were not significantly different (Table 1).
We further analyzed the relationship between hypocalcemia Perinatologyand factors, such as gestational age, small for gestational age, phosphorous, magnesium levels on day 1, and feeding amount by logistic regression. Only magnesium levels on day 1 were found to affect the risk for ENH (P=0.007), while other factors showed no significant effects (Table 2).
We determined that the receiver operating characteristic curve and magnesium levels on day 1 were related to hypocalcemia on day 3 with an odds ratio of 0.247 using a cutoff value of 1.7 mmol/L for hypocalcemia (sensitivity 42.6%, specificity 80.0%, P=0.005). However, it is inappropriate to use the cut-off value of 1.7 mmol/L to predict hypocalcemia due to its low sensitivity (Fig. 1).
We performed further analyses to evaluate whether the use of maternal magnesium or the cumulative dose of administered magnesium caused the newborns' magnesium levels on day 1. However, the analysis showed that both were unlikely to contribute to newborn magnesium levels on day 1 (P=0.239, P= 0.733, respectively).
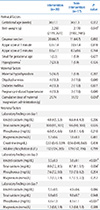
To study the effect of intervention, we compared the characteristics of the intervention and non-intervention groups (Table 3). Only birthweight, ionized calcium, and total serum calcium on day 3 were significantly different between the groups (P=0.045, P<0.001, and P=0.004, respectively). Compared to the non-intervention group, the intervention group showed a prominent increase in ionized calcium levels and total serum calcium levels from day 3 to day 7 (P=0.004, P=0.001, respectively). A rapid fall was seen in phosphorous levels from day 3 to day 7 in the intervention group (P<0.001). Magnesium was not affected by either calcium supplements or low-phosphorous formula feeding (P=0.237) (Fig. 2). In addition, all babies in the hypocalcemic group did not show any clinical seizures regardless of intervention.
ENH is not rare, even in moderate-to-late preterm infants, so all preterm infants should be observed for symptoms of hypocalcemia. The risk factors for ENH have been known for decades and include maternal diabetes, asphyxia, prematurity, and small for gestational age, among others. However, in this study that focused on moderate-to-late preterm infants, only newborn initial magnesium levels after birth were associated with ENH.
Lower magnesium levels on day 1 were related to lower calcium levels and increased the risk for hypocalcemia about 4-fold. The metabolic connection between calcium and magnesium is PTH, known to raise the plasma concentrations of both calcium and magnesium.2021 Magnesium is necessary for both PTH secretion and the peripheral responsiveness to PTH.11 Decreased PTH secretion due to hypomagnesemia may be partially counterbalanced by compensatory increases in PTH secretion in response to hypocalcemia caused by alterations in the responsiveness to PTH.11 Conversely, high levels of magnesium increase PTH, which causes higher serum calcium levels.
Hypermagnesemia at day 1 was further studied by analyzing its relationship with a history of maternal magnesium administration and its cumulative dose. Neonatal complications, such as hypotonia or bradycardia, are higher in infants whose mothers received magnesium for maternal indications,222324 which implies that neonatal magnesium levels are associated with maternal magnesium administration. However, in our study, there was no significant association between magnesium levels on day 1 and maternal magnesium administration or cumulative dose. Further studies, including laboratory findings of maternal serum magnesium levels before delivery, will be helpful to elucidate the relationship.
Numerous hormones, such as 1,25-dihydroxycholecalciferol (calcitriol), PTH, and calcitonin, also influence the metabolism of calcium and magnesium.25 Calcitriol increases the level of calcium in the blood by increasing the uptake of calcium from the gut, kidneys, and bone, while PTH increases the kidney excretion rate of phosphorous.26 PTH stimulates the production of calcitriol and calcitriol inhibits the release of calcitonin, which reduces blood calcium by inhibiting calcium release from bone.26 Since our study was designed to be retrospective, lack of such hormone data is a limitation that should be addressed in future studies. It also emphasizes the fact that the relationship between ENH and such hormonal effects should be studied further.
The benefits of intervention for ENH in moderate-to-late preterm infants has been doubted by many clinicians due to the lack of referential evidence. There has been a long ongoing debate about calcium supplementation for ENH and the effectiveness of oral calcium supplementation.2728 Thus, we additionally evaluated the effectiveness of interventions for ENH in moderate-to-late preterm infants. Of course, interventions with calcium supplements or low-phosphorous milk feeding were more effective in resolving the infants' hypocalcemia than no intervention. However, the non-intervention group also showed spontaneous recovery of serum calcium levels, although the difference between the calcium level changes were significant between the intervention and non-intervention groups. Changes in magnesium level are known to be much smaller than the accompanying changes in calcium concentration.20 This agrees with our study where changes in serum magnesium were not observed between the intervention and non-intervention groups. Further randomized controlled studies will be performed to confirm the efficacy of the interventions for hypocalcemia in moderate-to-late preterm infants.
The strength of our study was that it was based on moderate-to-late preterm infants, in whom ENH is commonly observed. Also, serial blood test results obtained throughout the week after birth were useful in detecting and comparing changes in laboratory findings. However, there were some limitations to our study. It was a small scale study, not a randomized controlled study, and lacked other laboratory findings because it was a retrospective study.
In conclusion, lower magnesium levels on day 1 were related to lower calcium levels that caused hypocalcemia. Moderate-to-late preterm infants with low magnesium levels on day 1 should be monitored carefully for ENH. Since the serum magnesium level is a delicate factor in predicting serum calcium levels, its predictive value for hypocalcemia is promising. Whether intervention is performed for ENH is still debatable because other factors should also be considered. Large-scale randomized controlled studies are required to investigate the clinical application of using infants' magnesium levels to predict hypocalcemia and to decide whether to perform interventions for ENH in moderate-to-late preterm infants.
Figures and Tables
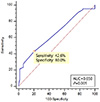 | Fig. 1ROC curve between newborn magnesium levels on day 1 and hypocalcemia on day 3. AUC, area under curves; ROC, receiver operating characteristic curves. |
 | Fig. 2Change in laboratory findings from newborn day 3 to day 7 and comparison of the significance between the intervention and non-intervention groups. P-value: significance of the change of values between two groups from day 3 to day 7. |
References
1. Jain A, Agarwal R, Sankar MJ, Deorari A, Paul VK. Hypocalcemia in the newborn. Indian J Pediatr. 2010; 77:1123–1128.

2. Picone S, Paolillo P. Neonatal outcomes in a population of late-preterm infants. J Matern Fetal Neonatal Med. 2010; 23:116–120.

3. Mayne PD, Kovar IZ. Calcium and phosphorus metabolism in the premature infant. Ann Clin Biochem. 1991; 28:131–142.

4. Kovacs CS. Calcium, phosphorus, and bone metabolism in the fetus and newborn. Early Hum Dev. 2015; 91:623–628.

5. Venkataraman PS, Tsang RC, Steichen JJ, Grey I, Neylan M, Fleischman AR. Early neonatal hypocalcemia in extremely preterm infants. High incidence, early onset, and refractoriness to supraphysiologic doses of calcitriol. Am J Dis Child. 1986; 140:1004–1008.
6. Bagnoli F, Bruchi S, Garosi G, Pecciarini L, Bracci R. Relationship between mode of delivery and neonatal calcium homeostasis. Eur J Pediatr. 1990; 149:800–803.

7. Brown JV, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst Rev. 2016; (5):CD000343.

8. Nelson N, Finnström O, Larsson L. Plasma ionized calcium, phosphate and magnesium in preterm and small for gestational age infants. Acta Paediatr Scand. 1989; 78:351–357.

9. Cloherty JP, Eichenwald EC, Hansen AR, Soul JS. Perinatal asphyxia and hypoxic-ischemic encephalopathy. Manual of neonatal care. 7th ed. Philadelphia: Lippincott Williams & Wilkins;2011. p. 711–728.
10. Rai S, Bhatiyani KK, Kaur S. Effect of birth asphyxia on serum calcium and glucose level: a prospective study. Int J Sci Study. 2015; 3:3–6.
11. Thomas TC, Smith JM, White PC, Adhikari S. Transient neonatal hypocalcemia: presentation and outcomes. Pediatrics. 2012; 129:e1461–e1467.

12. Tsang RC, Kleinman LI, Sutherland JM, Light IJ. Hypocalcemia in infants of diabetic mothers. Studies in calcium, phosphorus, and magnesium metabolism and parathormone responsiveness. J Pediatr. 1972; 80:384–395.
13. Bergman L, Kjellmer I, Selstam U. Calcitonin and parathyroid hormone-relation to early neonatal hypocalcemia in infants of diabetic mothers. Biol Neonate. 1974; 24:151–160.
14. Taneja A, Arora K, Chopra I, Naik SS. Pregnancy outcomes in isolated oligohydramnios during second trimester: a case series. J Clin Diagn Res. 2017; 11:QR01-QR02.

16. Dokos C, Tsakalidis C, Manaridou K, Karayianni P, Kyrkos I, Roussos I. Clinical-laboratory findings of bone metabolism in healthy premature and full-term neonates: preliminary results. Clin Cases Miner Bone Metab. 2017; 14:167–172.

17. Cockburn F, Brown JK, Belton NR, Forfar JO. Neonatal convulsions associated with primary disturbance of calcium, phosphorus, and magnesium metabolism. Arch Dis Child. 1973; 48:99–108.

18. Buppasiri P, Lumbiganon P, Thinkhamrop J, Ngamjarus C, Laopaiboon M, Medley N. Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes. Cochrane Database Syst Rev. 2015; (2):CD007079.

19. Harding JE, Wilson J, Brown J. Calcium and phosphorus supplementation of human milk for preterm infants. Cochrane Database Syst Rev. 2017; 2:CD003310.

20. Heaton FW. Magnesium relations with parathyroid hormone, calcitonin and bone. Magnes Bull. 1981; 1:67–72.
22. Ambadkar A, Prasad M, Chauhan AR. Neonatal effects of maternal magnesium sulphate in late preterm and term pregnancies. J Obstet Gynaecol India. 2019; 69:25–30.

23. Das M, Chaudhuri PR, Mondal BC, Mitra S, Bandyopadhyay D, Pramanik S. Assessment of serum magnesium levels and its outcome in neonates of eclamptic mothers treated with low-dose magnesium sulfate regimen. Indian J Pharmacol. 2015; 47:502–508.

24. Abbassi-Ghanavati M, Alexander JM, McIntire DD, Savani RC, Leveno KJ. Neonatal effects of magnesium sulfate given to the mother. Am J Perinatol. 2012; 29:795–799.

25. Baker SB, Worthley LI. The essentials of calcium, magnesium and phosphate metabolism: part I. Physiology. Crit Care Resusc. 2002; 4:301–306.
26. Voet D, Voet JG. Biomolecules, mechanisms of enzyme action, and metabolism. 1st ed. New York: Wiley;2004. p. 663–664.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download