Abstract
Late postpartum eclampsia is defined as the onset of a seizure more than 48 hours after delivery. Posterior reversible encephalopathy syndrome (PRES) is a clinical-radiological entity characterized by clinical manifestations such as seizures, headaches, visual disturbances, altered mental status, and characteristic magnetic resonance imaging findings, including sub-cortical vasogenic edema at the bilateral parietal and occipital lobes. Late postpartum eclampsia concurrent with PRES is rare, and not always preceded by preeclampsia before delivery. We report the case of a 26-year-old woman who developed PRES with late postpartum eclampsia 5 days after cesarean delivery but did not have hypertensive disease during pregnancy.
Preeclampsia is defined as de novo hypertension developing after 20 weeks of gestation combined with one or more of the following new-onset conditions: proteinuria (spot urine protein/creatinine ≥0.3 mg/mg or ≥300 mg/day or at least 1 g/L [‘2+’] on dipstick testing), other maternal organ dysfunction such as renal insufficiency, liver involvement, neurological or hematological complications, and uteroplacental dysfunction.1 Eclampsia is defined as the occurrence of a seizure with preeclampsia. Preeclampsia and eclampsia usually occur between 20 weeks of gestation and 48 hours postpartum. Late postpartum eclampsia (LPE) comprises eclamptic events that develop more than 48 hours postpartum.2 Posterior reversible encephalopathy syndrome (PRES) was first described by Hinchey et al.3 in 1996. It is a rare and reversible neurological entity, characterized by clinical manifestations such as seizures, headaches, visual disturbances, altered mental status, and characteristic neuroimaging features.4 Magnetic resonance imaging (MRI) of PRES shows bilateral and symmetrical vasogenic edema involving cortical and sub-cortical lesions at the bilateral parietal and occipital lobes; however, some patients present with atypical distributions, such as in the anterior cerebral lobes, cerebellum, basal ganglia, and brain stem.56789 Various conditions and predisposing factors are associated with PRES, with the most common being hypertension and preeclampsia/eclampsia.10 Recent studies have shown that almost all patients with eclampsia also develop PRES.11 LPE concurrent with PRES is uncommon, and the exact prevalence is unknown. However, a recent study suggested it could be more common than expected.12 Unfortunately, most obstetricians have a limited awareness of this entity. Because early recognition and treatment are important to the outcome of PRES, clinicians should become familiar with the prodromal symptoms and clinical-radiological presentation. Misdiagnosis and delayed treatment may cause irreversible brain damage or even death. We report a rare case of PRES that developed with late postpartum eclampsia 5 days after cesarean delivery.
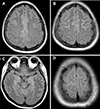
A 26-year-old woman (gravida 1, para 1) was admitted to the emergency department with severe headache and high blood pressure (180/113 mmHg). She did not have any complications or hypertensive disease during pregnancy, and she had no history of systemic disease or medication use. She underwent cesarean delivery due to cephalopelvic disproportion. On postpartum day 5, the patient experienced a sudden headache and high blood pressure. In the emergency department, she experienced a generalized tonic-clonic seizure for 90 seconds, with left eyeball deviation, convulsion of the limbs, and cyanotic lips. Her temperature was 36.5℃. Intravenous lorazepam (2 mg) was administered immediately. High blood pressure was controlled with labetalol and nicardipine. A neurological examination indicated a somnolent state without any focal neurological signs. She also reported visual disturbance (blurred vision). Emergency laboratory blood tests showed the following: leukocytosis (white blood cell count, 14,610 cells/mm3); creatinine (1.37) higher than the normal range (0.6–1.2); and lactate dehydrogenase (315 U/L) slightly above the normal range (129–240 U/L). Hemoglobin, hematocrit, platelet count, blood glucose, serum electrolyte, liver function, and lactic acid levels were normal. Urinary dipstick confirmed no proteinuria. Computed tomography of the brain showed no acute intracranial hemorrhage. MRI of the brain was performed and indicated multifocal T2/fluid attenuated inversion recovery (FLAIR) hyperintense lesions involving both fronto-temporo-parieto-occipital cortices, both basal ganglia, the left thalamus, and both cerebellum, suggestive of PRES (Fig. 1). After admission to the Department of Neurology, a lumbar puncture was performed; cerebrospinal fluid protein, glucose, immunoglobulin, and the number of cells were found to be normal. Laboratory results showed creatinine (0.99, decreased), and urinary analysis showed proteinuria traces. The second day after admission, the patient experienced one more generalized tonic-clonic seizure. Soon after resolution of the seizures, while the patient was regaining consciousness, she reported left-side visual field symptoms (anopsia). Elevated blood pressure was recurrently measured. She experienced one more seizure. Magnesium sulfate and antihypertensive therapy for hypertension were administered after it was determined that she had LPE. Magnesium sulfate was started with a 4-g intravenous bolus, followed by a continuous drip of 1 g/hour to relieve eclamptic symptoms. Blood pressure normalized and symptoms subsided markedly over the course of the following days. On day 10 after admission, she was discharged home with improvements in symptoms and signs. Follow-up brain MRI performed after 3 months showed markedly decreased lesions (Fig. 2), and her blood pressure was normal.
LPE is distinguished from early postpartum eclampsia by its onset, which occurs more than 48 hours after childbirth. PRES associated with LPE is rare and can be easily misdiagnosed because of delayed onset and an atypical clinical presentation. The differential diagnosis for frequent convulsions during the puerperal period includes ischemic stroke, encephalitis, meningitis, Hashimoto's encephalopathy, mitochondrial encephalopathy, electrolyte or endocrine disturbances, vasculitis, inflammatory demyelinating disease, and neoplastic diseases.13 Laboratory tests, cerebrospinal fluid examinations, reversal of the brain MRI features following treatment, and good outcomes exclude the aforementioned differential diagnoses. In this case, the patient presented with LPE 5 days after delivery. She did not have preeclampsia before delivery. The initial evaluation in the emergency department did not reveal proteinuria. The diagnosis of PRES associated with LPE was based on her increased blood pressure, MRI findings suggestive of eclamptic PRES, and recent history of childbirth. For preeclampsia-/eclampsia-induced PRES, control of hypertension and prevention or treatment of seizures are essential. Magnesium sulfate is the drug of choice for epileptic seizures.14 In this case, our patient had recurrent convulsions, and the seizures were controlled by starting magnesium sulfate. Early diagnosis and adequate treatment are important for preventing permanent neurological complications. Although more than 90% of LPE patients had at least one prodromal symptom suggesting preeclampsia, less than 22% of them had been previously diagnosed with preeclampsia.15 This result indicates that eclampsia can be prevented if the patient goes to the hospital and is promptly diagnosed when preeclampsia symptoms develop. Considering the significant morbidity associated with LPE, clinicians should recognize the prodromal symptoms, be suspicious when the setting involves the recent postpartum period, and evaluate for PRES with a brain MRI, even without proteinuria. Health care providers should educate patients regarding the need to report all prodromal symptoms (headache, visual symptoms, nausea or vomiting, and epigastric pain) after delivery and after discharge from the hospital. Furthermore, they should inform patients that they could still be at risk for preeclampsia and eclampsia after delivery.
Figures and Tables
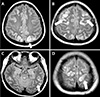 | Fig. 1Axial magnetic resonance imaging in the posterior reversible encephalopathy syndrome patient. T2/fluid attenuated inversion recovery images at various brain levels. (A–D) Multiple high signal-intensity lesions (arrows) in bilateral occipito-parietal regions, bilateral frontal and temporal lobes. |
References
1. Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014; 4:97–104.

2. Lubarsky SL, Barton JR, Friedman SA, Nasreddine S, Ramadan MK, Sibai BM. Late postpartum eclampsia revisited. Obstet Gynecol. 1994; 83:502–505.

3. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996; 334:494–500.

4. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015; 14:914–925.

5. Karampekios SK, Contopoulou E, Basta M, Tzagournissakis M, Gourtsoyiannis N. Hypertensive encephalopathy with predominant brain stem involvement: MRI findings. J Hum Hypertens. 2004; 18:133–134.

6. Choh NA, Jehangir M, Rasheed M, Mira T, Ahmad I, Choh S. Involvement of the cervical cord and medulla in posterior reversible encephalopathy syndrome. Ann Saudi Med. 2011; 31:90–92.

7. Chen TY, Lee HJ, Wu TC, Tsui YK. MR imaging findings of medulla oblongata involvement in posterior reversible encephalopathy syndrome secondary to hypertension. AJNR Am J Neuroradiol. 2009; 30:755–757.

8. Ono Y, Manabe Y, Hamakawa Y, Murakami T, Omori N, Hayashi Y, et al. Localized lesions on MRI in a case of hypertensive brainstem encephalopathy. Intern Med. 2005; 44:1002–1005.

9. Ekawa Y, Shiota M, Tobiume T, Shimaoka M, Tsuritani M, Kotani Y, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012; 226:55–58.

10. Servillo G, Bifulco F, De Robertis E, Piazza O, Striano P, Tortora F, et al. Posterior reversible encephalopathy syndrome in intensive care medicine. Intensive Care Med. 2007; 33:230–236.

11. Brewer J, Owens MY, Wallace K, Reeves AA, Morris R, Khan M, et al. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol. 2013; 208:468.e1–468.e6.

12. Wagner SJ, Acquah LA, Lindell EP, Craici IM, Wingo MT, Rose CH, et al. Posterior reversible encephalopathy syndrome and eclampsia: pressing the case for more aggressive blood pressure control. Mayo Clin Proc. 2011; 86:851–856.

13. Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005; 105:402–410.

14. Which anticonvulsant for women with eclampsia? Evidence from the collaborative eclampsia trial. Lancet. 1995; 345:1455–1463.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download