Abstract
Purpose
To investigate the effects of hydrogen sulfide (H2 S) on the permeability of a cultured human trabecular meshwork cells (HTMC) monolayer and its interaction with nitric oxide (NO).
Methods
After exposing primary cultured HTMCs to 0, 50, 100, and 500 μ M sodium hydrogen sulfide (NaHS) for 6 hours, the permeabilities through the HTMC monolayer were measured using a Transwell assay with carboxyfluorescein. The production of NO and eNOS mRNA expression were assessed using the Griess assay and reverse transcription-polymerase chain reaction, respectively. In addition, 0, 1, and 10 μ M NaHS and 10 μ M sodium nitroprusside (SN) were co-exposed to evaluate the possible synergistic effect of H2 S and NO.
Results
Greater than 100 μ M NaHS increased the permeability through the HTMC monolayer in a dose-dependent manner (p < 0.05). These increased permeabilities were not accompanied by NO production or eNOS mRNA expression (p > 0.05). When 0, 1, and 10 μ M NaHS and 10 μ M SN were exposed together, there was no significant change of permeability, NO production, or eNOS mRNA expression (all, p > 0.05).
Go to : 
References
1. Ł owicka E, Beł towski J. Hydrogen sulfide (H2 S) – the third gas of interest for pharmacologists. Pharmacol Rep. 2007; 59:4–24.
2. Chen CQ, Xin H, Zhu YZ. Hydrogen sulfide: third gaseous trans-mitter, but with great pharmacological potential. Acta Pharmacol Sin. 2007; 28:1709–16.

3. Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011; 51:169–87.

4. Kabil O, Banerjee R. Enzymology of H2 S biogenesis, decay and signaling. Antioxid Redox Signal. 2014; 20:770–82.
5. Kimura H. Production and physiological effects of hydrogen sulfide. Antioxid Redox Signal. 2014; 20:783–93.

6. Elsey DJ, Fowkes RC, Baxter GF. Regulation of cardiovascular cell function by hydrogen sulfide (H2 S). Cell Biochem Funct. 2010; 28:95–106.
7. Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004; 18:1165–7.

8. Wallace JL. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol Sci. 2007; 28:501–5.

9. Zhang Y, Tang ZH, Ren Z, et al. Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol Cell Biol. 2013; 33:1104–13.

10. Kulkarni M, Njie-Mbye YF, Okpobiri I, et al. Endogenous production of hydrogen sulfide in isolated bovine eye. Neurochem Res. 2011; 36:1540–5.

11. Goldblum D, Mittag T. Prospects for relevant glaucoma models with retinal ganglion cell damage in the rodent eye. Vision Res. 2002; 42:471–8.

12. Lu M, Hu LF, Hu G, Bian JS. Hydrogen sulfide protects astrocytes against H2 O2- induced neural injury via enhancing glutamate uptake. Free Radic Biol Med. 2008; 45:1705–13.
13. Sakamoto K, Suzuki Y, Kurauchi Y, et al. Hydrogen sulfide abdominal NMDA-induced neuronal injury via its anti-oxidative activity in the rat retina. Exp Eye Res. 2014; 120:90–6.
14. Liu H, Anders F, Thanos S, et al. Hydrogen sulfide protects retinal ganglion cells against glaucomatous injury in vitro and in vivo. Invest Ophthalmol Vis Sci. 2017; 58:5129–41.

15. Huang S, Huang P, Lin Z, et al. Hydrogen sulfide supplement attenuates the apoptosis of retinal ganglion cells in experimental glaucoma. Exp Eye Res. 2018; 168:33–48.

16. Salvi A, Bankhele P, Jamil J, et al. Effect of hydrogen sulfide abdominals on intraocular pressure in rabbits. J Ocul Pharmacol Ther. 2016; 32:371–5.
17. Robinson J, Okoro E, Ezuedu C, et al. Effects of hydrogen sul-fide-releasing compounds on aqueous humor outflow facility in porcine ocular anterior segments, Ex Vivo. J Ocul Pharmacol Ther. 2017; 33:91–7.

18. Kida M, Sugiyama T, Yoshimoto T, Ogawa Y. Hydrogen sulfide abdominals nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells. Eur J Pharm Sci. 2013; 48:211–5.
19. Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997; 237:527–31.

20. Bruce King S. Potential biological chemistry of hydrogen sulfide (H2 S) with the nitrogen oxides. Free Radic Biol Med. 2013; 55:1–7.
21. Nagpure BV, Bian JS. Interaction of hydrogen sulfide with nitric oxide in the cardiovascular system. Oxid Med Cell Longev. 2016; 2016:6904327.

22. Mosmann T. Rapid colorimetric assay for cellular growth and abdominal: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
23. Fremoser FM, Jakob CA, Aebi M, Tuor U. The MTT [3-(4,5-dime-thylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl Environ Microbio. 1999; 65:3727–9.
24. Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrites and nitrates in biological fluids. Analytical Biochem. 1982; 126:131–8.
25. Grimes PA, Stone RA, Laties AM, Li W. Carboxyfluorescein. A probe of the blood-ocular barriers with lower membrane abdominal than fluorescein. Arch Ophthalmol. 1982; 100:635–9.
26. Araie M. Carboxyfluorescein. A dye for evaluating the corneal abdominal barrier function in vivo. Exp Eye Res. 1986; 42:141–50.
27. Grimes PA. Carboxyfluorescein transfer across the blood-retinal barrier evaluated by quantitative fluorescence microscopy: abdominal with fluorescein. Exp Eye Res. 1988; 46:769–83.
28. Nakagawa S, Usui T, Yokoo S, et al. Toxicity evaluation of abdominal drugs using stratified human cultivated corneal abdominal sheets. Invest Ophthalmol Vis Sci. 2012; 53:5154–60.
29. Lei Y, Stamer WD, Wu J, Sun X. Oxidative stress impact on barrier function of porcine angular aqueous plexus cell monolayers. Invest Ophthalmol Vis Sci. 2013; 54:4827–35.

30. Abe K, Kimura H. The possible role of hydrogen sulfide as an abdominal neuromodulator. J Neurosci. 1996; 16:1066–71.
33. Persa C, Osmotherly K, Chao-Wei Chen K, et al. The distribution of cystathionine beta-synthase (CBS) in the eye: implication of the presence of a trans-sulfuration pathway for oxidative stress defense. Exp Eye Res. 2006; 83:817–23.
34. Wiederholt M, Dörschner N, Groth J. Effect of diuretics, channel modulators, and signal interceptors on contractility of the abdominal meshwork. Ophthalmologica. 1997; 211:153–61.
35. Wiederholt M, Stumpff F. The trabecular meshwork and aqueous humor reabsorption. Civan MM, editor. Current topics in membranes. The eye's aqueous Humor: from secretion to glaucoma. 1st ed.San Diego: Academic Press;1998. p. 163–202.
36. Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of abdominal meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994; 35:2515–20.
37. Behar-Cohen FF, Goureau O, D'Hermies F, Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest Ophthalmol Vis Sci. 1996; 37:1711–5.
38. Dismuke WM, Mbadugha CC, Ellis DZ. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am J Physiol Cell Physiol. 2008; 294:C1378–86.

39. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO. 2001; 20:6008–16.

40. Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2 S in rat tissues. Can J Physiol Pharmacol. 2003; 81:848–53.
41. Kubo S, Doe I, Kurokawa Y, et al. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution to dual modulation of vascular tension. Toxicology. 2007; 232:138–46.

42. Geng B, Cui Y, Zhao J, et al. Hydrogen sulfide downregulates the aortic L-arginine/nitric oxide pathway in rats. Am J Physiol Regul Integr Comp Physiol. 2007; 293:R1608–18.

43. Yong QC, Hu LF, Wang S, et al. Hydrogen sulfide interacts with nitric oxide in the heart: possible involvement of nitroxyl. Cardiovasc Res. 2010; 88:482–91.

44. Wu D, Hu Q, Zhu D. An update on hydrogen sulfide and nitric abdominal interactions in the cardiovascular system. Oxid Med Cell Longev. 2018; 2018:4579140.
45. Roedl JB, Bleich S, Reulbach U, et al. Homocystein levels in aqueous humor and plasma of patients with primary open-angle glaucoma. J Neural Transm (Vienna). 2007; 114:445–50.
46. Roedl JB, Bleich S, Reulbach U, et al. Homocysteine in tear fluid of patients with pseudoexfoliation glaucoma. J Glaucoma. 2007; 16:234–9.

47. Patil A, Singh S, Opere C, Dash A. Sustained-release delivery abdominal of a slow hydrogen sulfide donor, GYY 4137, for potential application in glaucoma. AAPS PharmSciTech. 2017; 18:2291–302.
Go to : 
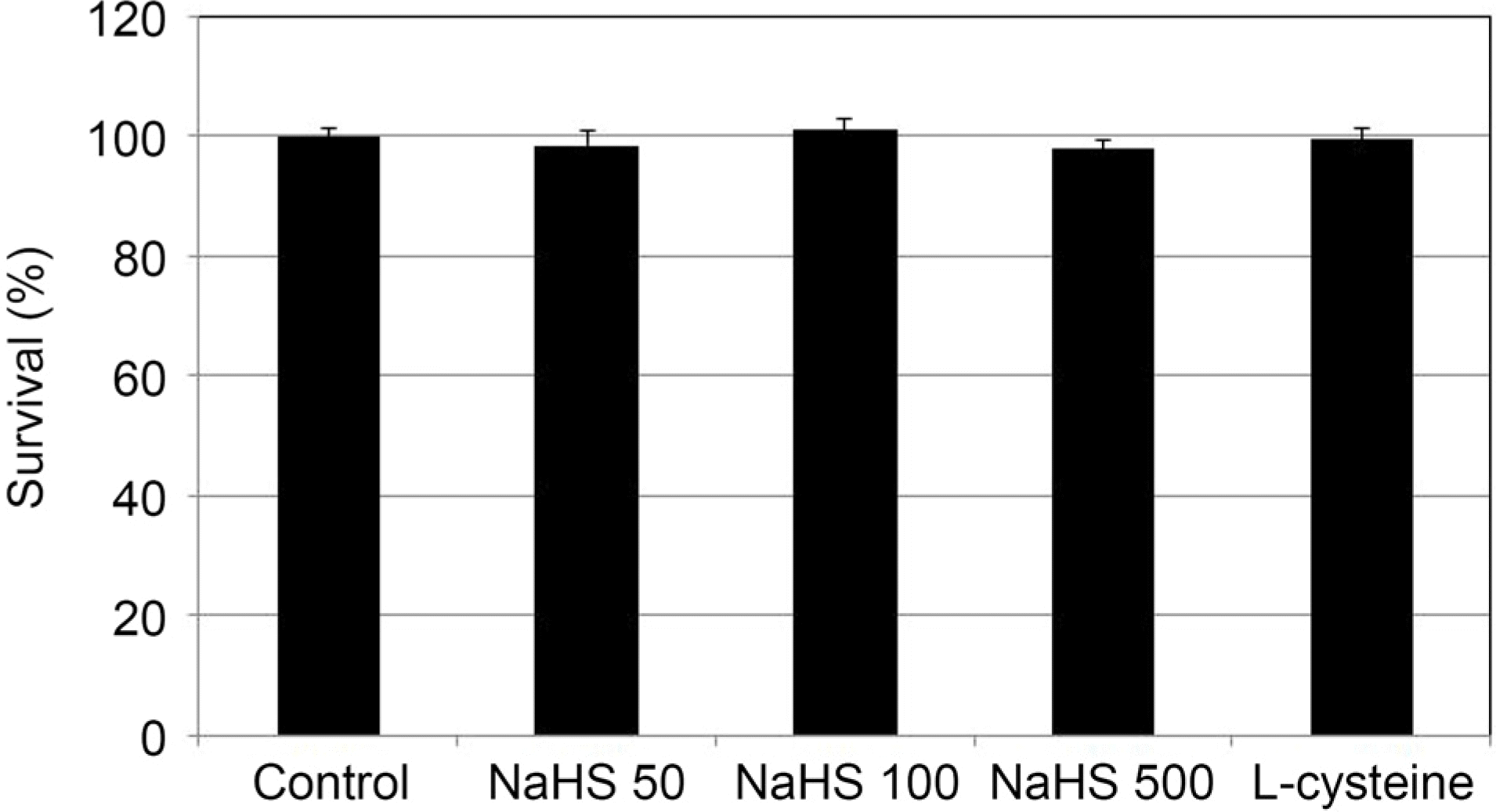 | Figure 1.The Effect of sodium hydrogen sulfide (NaHS; 0, 50,100, 500 μ M) and L-cysteine (100 μ M) on the survival of cultured human trabecular meshwork cells. Both NaHS and L-cys-teine did not affect the survival of trabecular meshwork cells compared to non-exposed controls (all p > 0.05). |
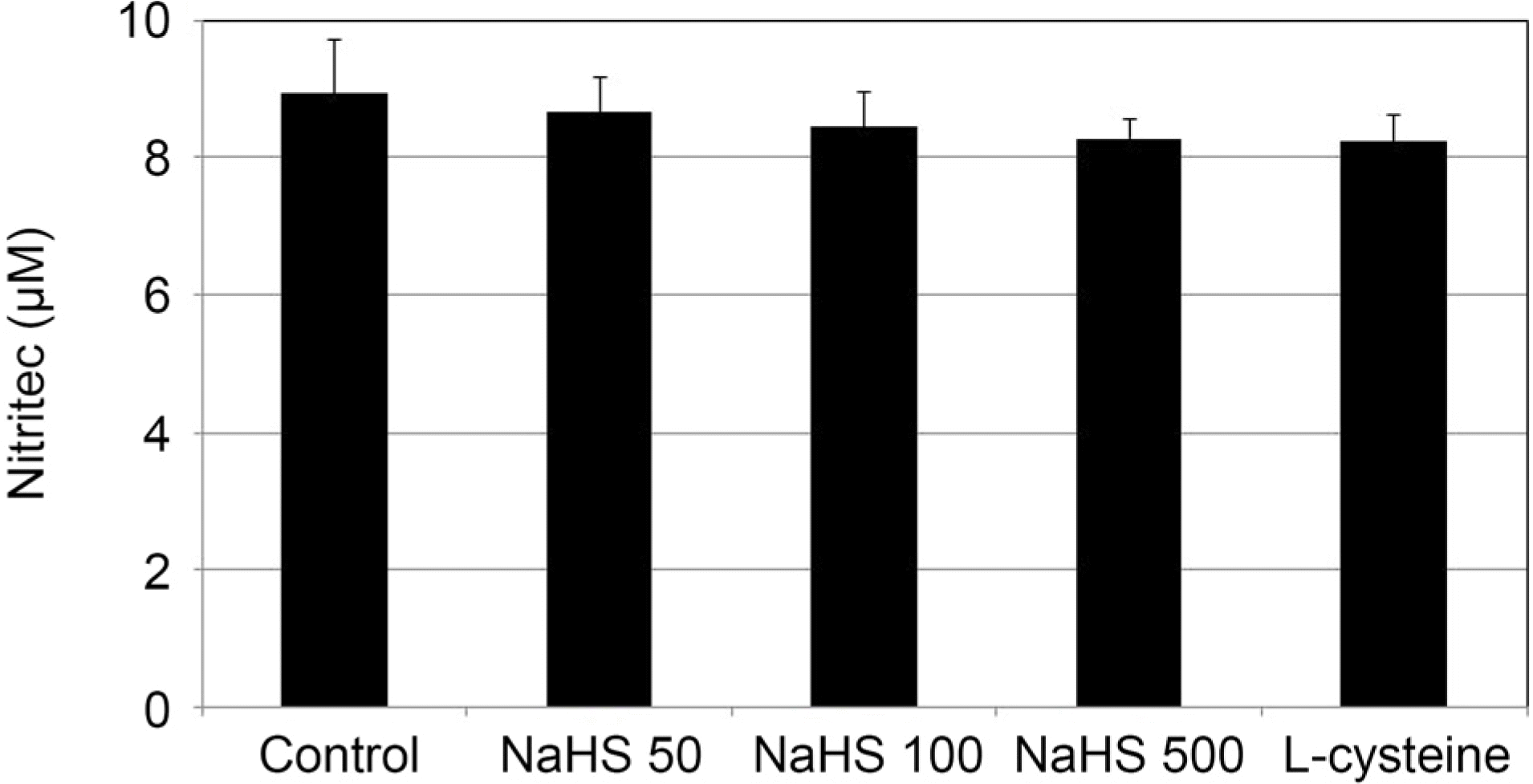 | Figure 2.The Effect of sodium hydrogen sulfide (NaHS; 0, 50,100, 500 μ M) and L-cysteine (100 μ M) on the production of nitric oxide in cultured human trabecular meshwork cells. Both NaHS and L-cysteine did not affect the production of nitric oxide compared to non-exposed controls (all p > 0.05). |
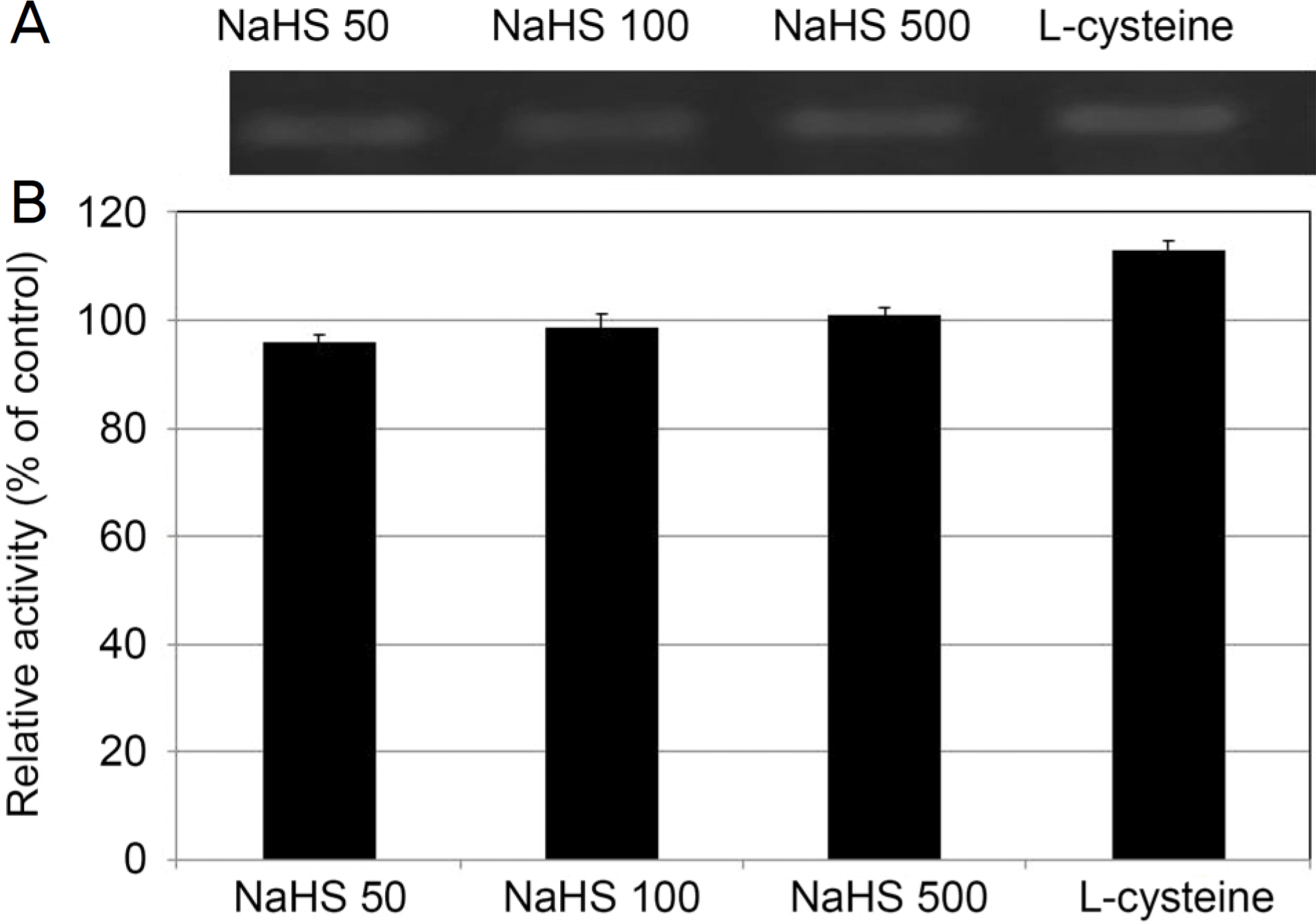 | Figure 3.The Effect of sodium hydrogen sulfide (NaHS; 0, 50, 100, 500 μ M) and L-cysteine (100 μ M) on the expression of endothelial nitric oxide synthase (eNOS) mRNA in cultured human trabecular meshwork cells. Both NaHS and L-cysteine did not affect the on the expression of eNOS mRNA compared to non-exposed controls (all p > 0.05). |
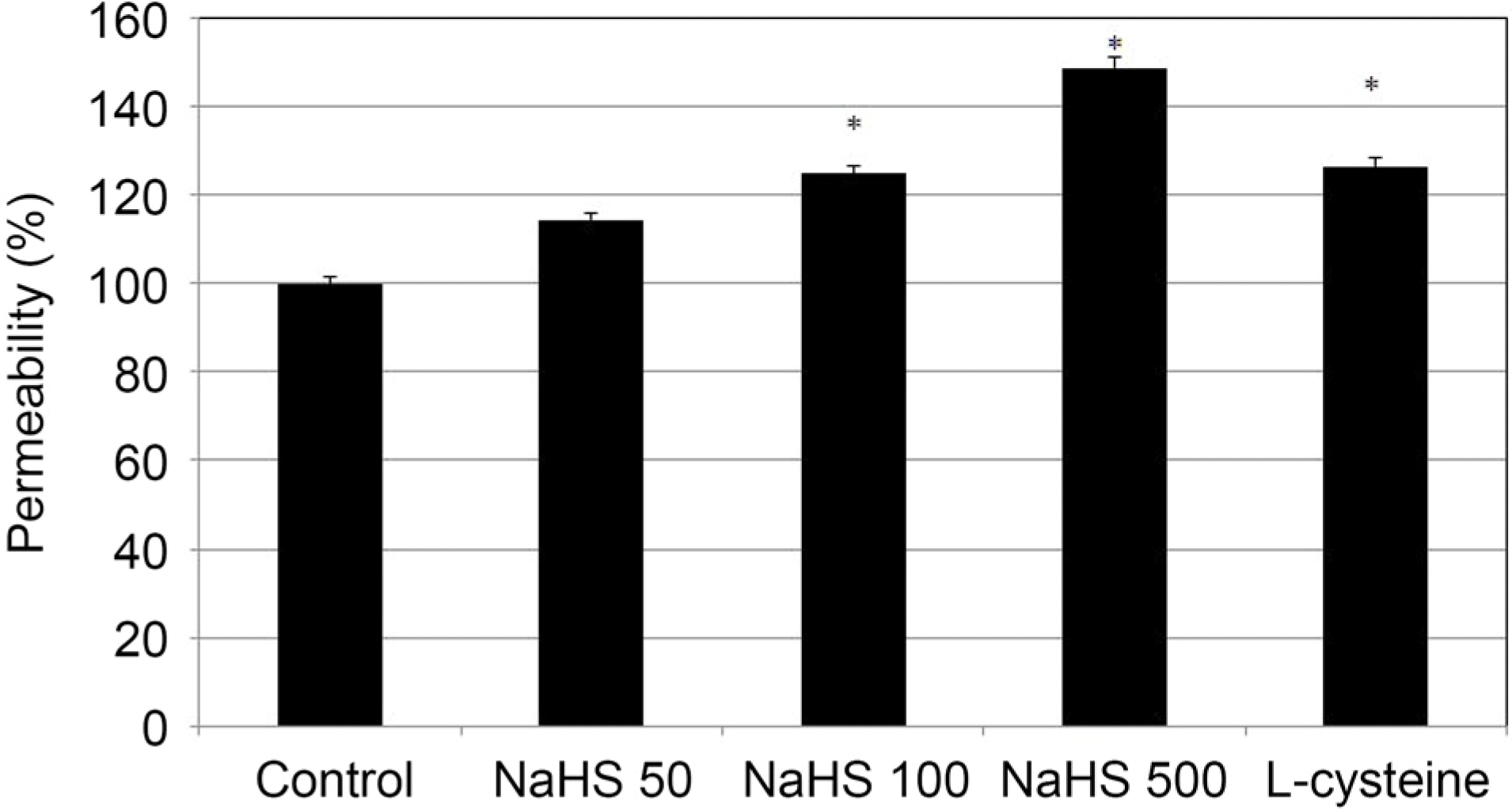 | Figure 4.The Effect of sodium hydrogen sulfide (NaHS: 0, 50,100, 500 μ M) and L-cysteine (100 μ M) on the permeability of carboxyfluorescin through the trabecular meshwork cell monolayer. Both 100, 500 μ M NaHS and 100 μ M L-cysteine increased the permeabilty of carboxyfluorescein significantly (* p < 0.05). Carboxyfluorescein intensity of outer chamber normalized to the mean value obtained using non-exposed control (permeability 100%). |
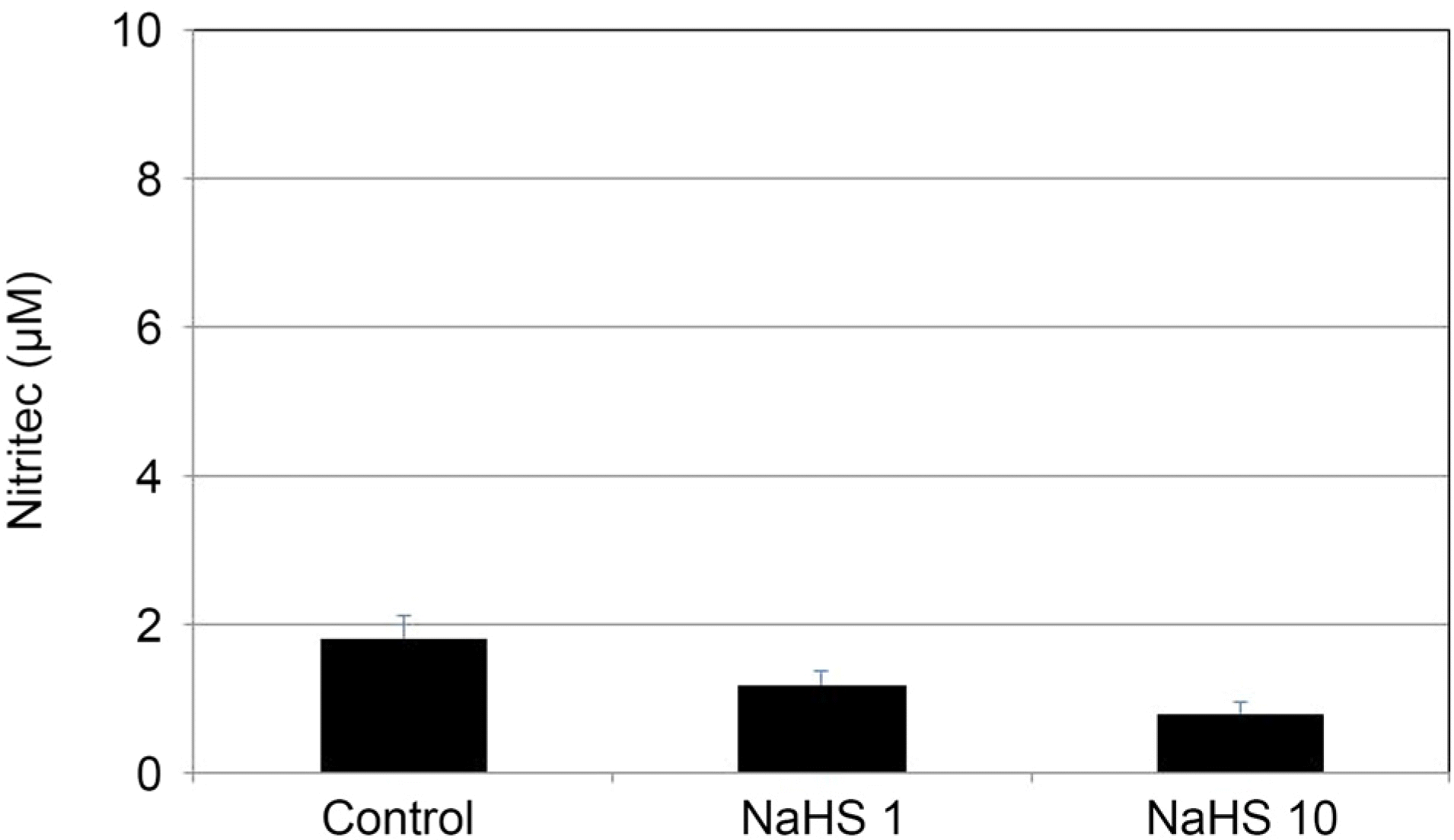 | Figure 5.The Effect of sodium hydrogen sulfide (NaHS) at low concentration (1, 10 μ M) on the production of nitric oxide in cultured human trabecular meshwork cells. NaHS at low concentration did not affect the production of nitric oxide (all p >0.05). |
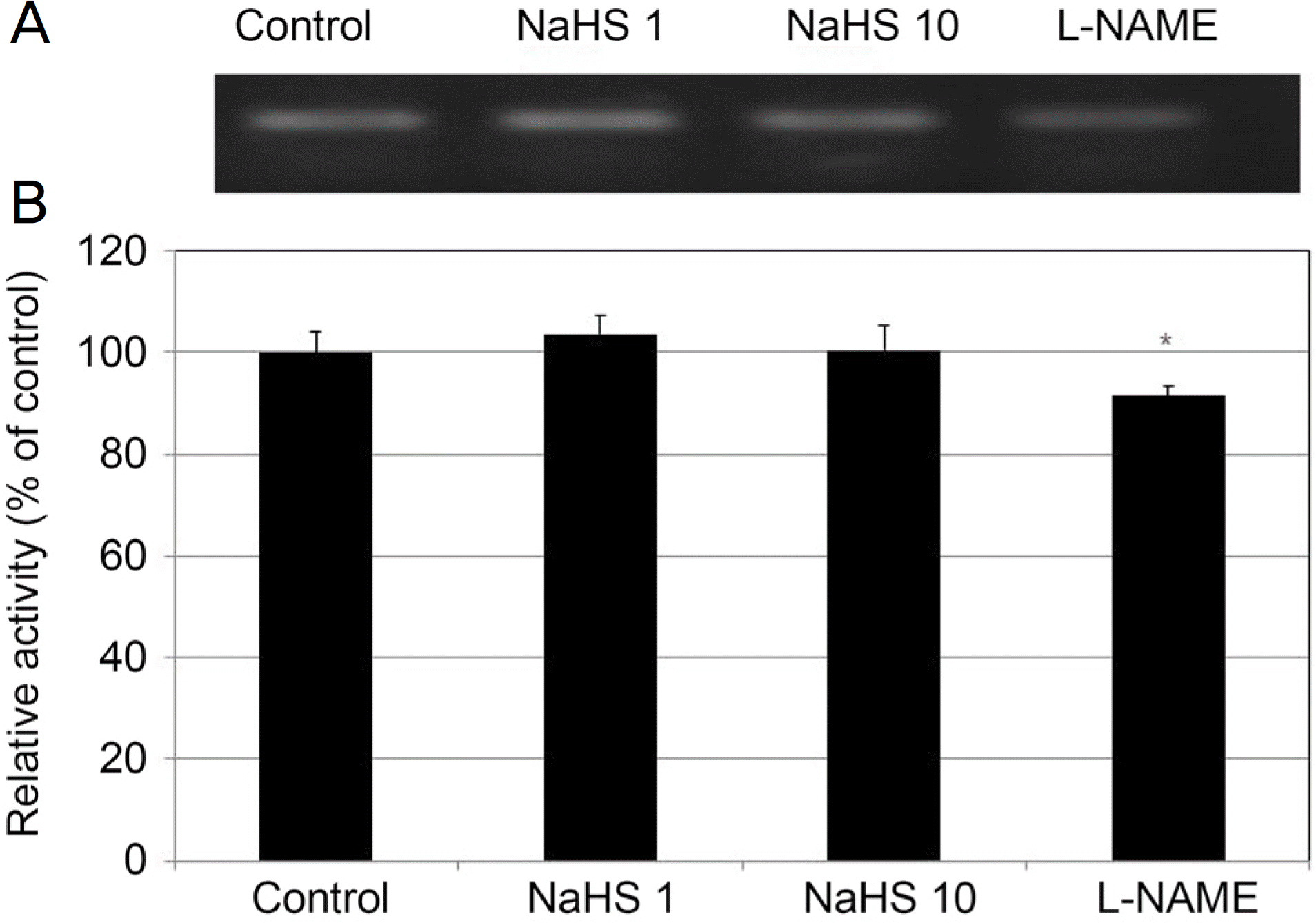 | Figure 6.Effect of sodium hydrogen sulfide (NaHS) at low concentration (1, 10 μ M) and Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME, 10 μ M) on the expression of endothelial nitric oxide synthase (eNOS) mRNA in cultured human trabecular meshwork cells. NaHS did not affect the on the expression of eNOS mRNA compared to non-exposed controls (all p > 0.05). In contrast, L-NAME decreased the expression of eNOS mRNA significantly (p = 0.046). |
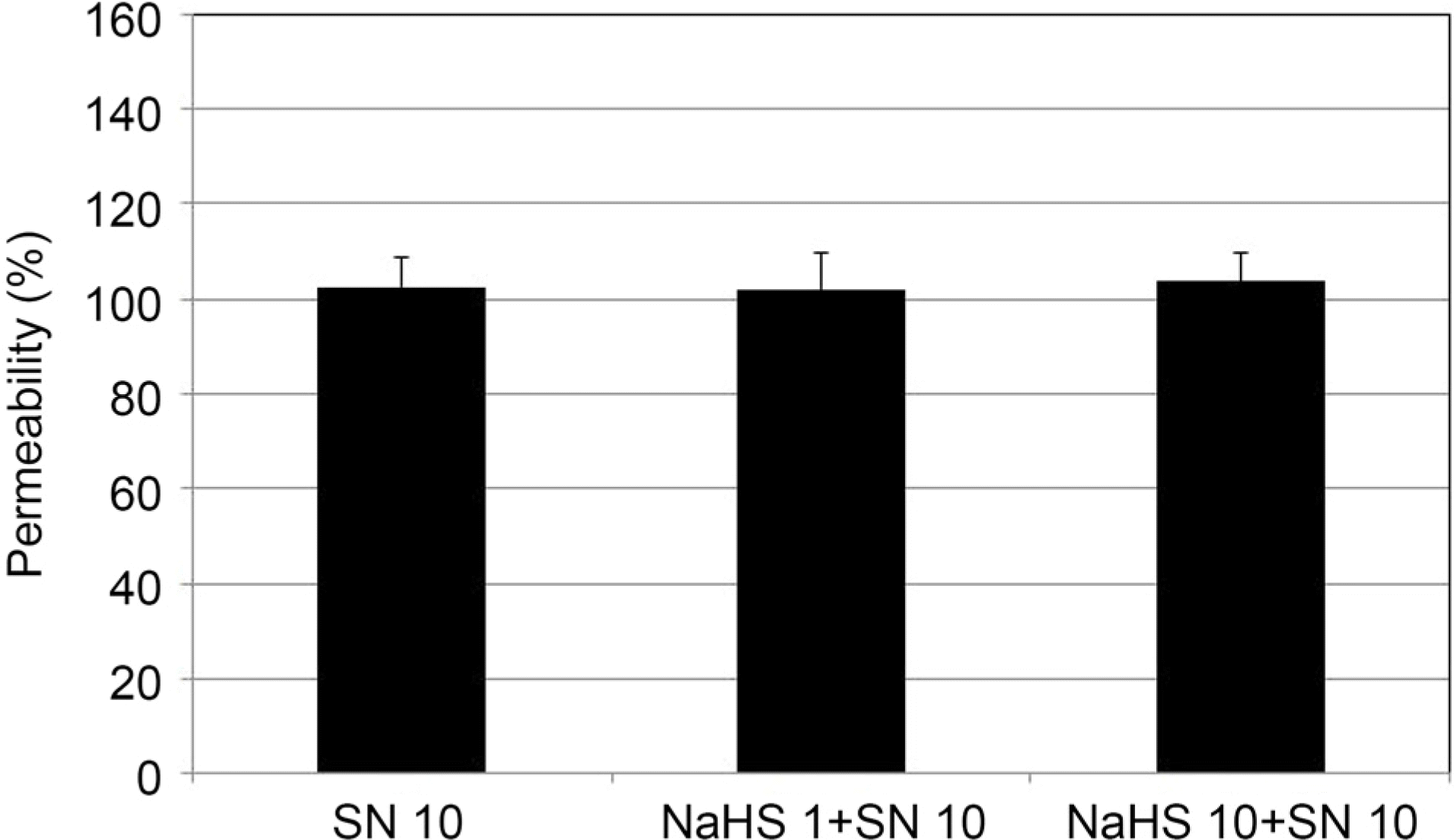 | Figure 7.Effect of 10 μ M sodium nitroprusside (SN) co-ex-posed to sodium hydrogen sulfide (NaHS) at low concentration (1, 10 μ M) on the permeability of carboxyfluorescein through the trabecular meshwork cell monolayer. Both 1, 10 μ M NaHS co-exposed with SN did not affect the permeabilty of carboxyfluorescein (p = 0.74, p = 0.511) compared with exposed to SN alone. Carboxyfluorescein intensity of outer chamber normalized to the mean value obtained using SN (permeability 100%). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download