Abstract
Purpose
To evaluate the effect of oral spironolactone for nonresolving chronic central serous chorioretinopathy after intravitreal bevacizumab injection.
Methods
Seventeen eyes of 17 patients with nonresolving chronic central serous chorioretinopathy after intravitreal bevacizumab injection from September 2017 to December 2018 were treated with oral spironolactone for 6 months, and changes in central macular thickness, subretinal fluid height, and best-corrected visual acuity (BCVA) were analyzed retrospectively.
Results
The central macular thickness decreased from 309.94 ± 105.20 µm at baseline to 259.76 ± 81.83 µm at 3 months, and 243.11 ± 61.98 µm at 6 months, which were both statistically significant (Wilcoxon signed-rank test, p = 0.016 and p = 0.001, respectively). The subretinal fluid height decreased from 138.05 ± 95.69 µm at baseline to 70.88 ± 83.13 µm at 3 months, and 54.00 ± 56.25 µm at 6 months, which were both statistically significant (Wilcoxon signed-rank test, p = 0.002 and p = 0.000, respectively). The BCVA (LogMAR) changed from 0.30 ± 0.38 at baseline to 0.35 ± 0.43 at 1 month, 0.29 ± 0.43 at 3 months, and 0.26 ± 0.40 at 6 months. The results at 6 months were statistically significant (Wilcoxon signed-rank test, p = 0.033). There were no side effects in patients treated with oral spironolactone.
Go to : 
References
1. Gergely R, Kovács I, Schneider M, et al. Mineralocorticoid abdominal antagonist treatment in bilateral chronic central serous abdominal: a comparative study of exudative and nonexudative fellow eyes. Retina. 2017; 37:1084–91.
2. Kitzmann AS, Pulido JS, Diehl NN, et al. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology. 2008; 115:169–73.

3. Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous abdominal: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013; 41:201–14.
4. Herold TR, Prause K, Wolf A, et al. Spironolactone in the treatment of central serous chorioretinopathya case series. Graefes Arch Clin Exp Ophthalmol. 2014; 252:1985–91.
5. Herold TR, Rist K, Priglinger SG, et al. abdominal results and abdominal rates after spironolactone treatment in nonresolving abdominal serous abdominal (CSCR). Graefes Arch Clin Exp Ophthalmol. 2017; 255:221–9.
6. Kapoor KG, Wagner AL. Mineralocorticoid antagonists in the treatment of central serous chorioretinopathy: a comparative analysis. Ophthalmic Res. 2016; 56:17–22.

7. Kweon EY. Factors influencing the effect of the intravitreal abdominal injection in patients with central serous chorioretinopathy. J Korean Opthalmol Soc. 2014; 55:391–5.
8. Koh KM, Kim JY, Kim JW, Choi MJ. The efficacy of intravitreal bevacizumab injection in patients with acute central serous chorioretinopathy. J Korean Opthalmol Soc. 2012; 53:781–5.

9. Yeo YD, Kim JH, Kim YC, Kim KS. Photodynamic therapy and focal laser photocoagulation in chronic central serous choriretinopathy. J Korean Opthalmol Soc. 2016; 57:56–62.
10. Kim YS, Lee YH, Kim HS, et al. Comparison of therapeutic effect between half-energy photodynamic therapy and intravitreal abdominal injection in chronic central serous chorioretinopathy for 12 months. J Korean Opthalmol Soc. 2013; 54:1526–33.
11. Falavarjani KG, Amirsardari A, Habibi A, et al. Visual and abdominalal outcomes of spironolactone therapy in patients with chronic central serous chorioretinopathy. J Ophthalmic Vis Res. 2017; 12:281–9.
12. Zhao M, Célérier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012; 122:2672–9.

13. Zhao M, Valamanesh F, Celerier I, et al. The neuroretina is a novel mineralocorticoid target: aldosterone upregulates ion and water channels in Müller glial cells. FASEB J. 2010; 24:3405–15.

14. Ghadiali Q, Jung JJ, Yu S, et al. Central serous chorioretinopathy treated with mineralocorticoid antagonists: a one-year pilot study. Retina. 2016; 36:611–8.
15. Sun X, Shuai Y, Fang W, et al. Spironolactone versus observation in the treatment of acute central serous chorioretinopathy. Br J Ophthalmol. 2018; 102:1060–5.

16. Kim JA, Shin JY, Bae SH, et al. Comparison of choroidal abdominal change after photodynamic therapy and ranibizumab for chronic central serous chorioretinopathy. J Korean Opthalmol Soc. 2015; 56:205–12.
17. Park SY, Koh HJ, Kim YM. Correlation between optical coherence tomography and visual-acuity in chronic central serous abdominal treated with half dose photodynamic therapy. J Korean Opthalmol Soc. 2013; 54:1715–22.
18. Bousquet E, Beydoun T, Zhao M, et al. Mineralocorticoid receptor antagonism in the treatment of chronic central serous abdominal: a pilot study. Retina. 2013; 33:2096–102.
19. Bousquet E, Beydoun T, Rothschild PR, et al. Spironolactone for nonresolving central serous chorioretinopathy: a randomized abdominalled crossover study. Retina. 2015; 35:2505–15.
Go to : 
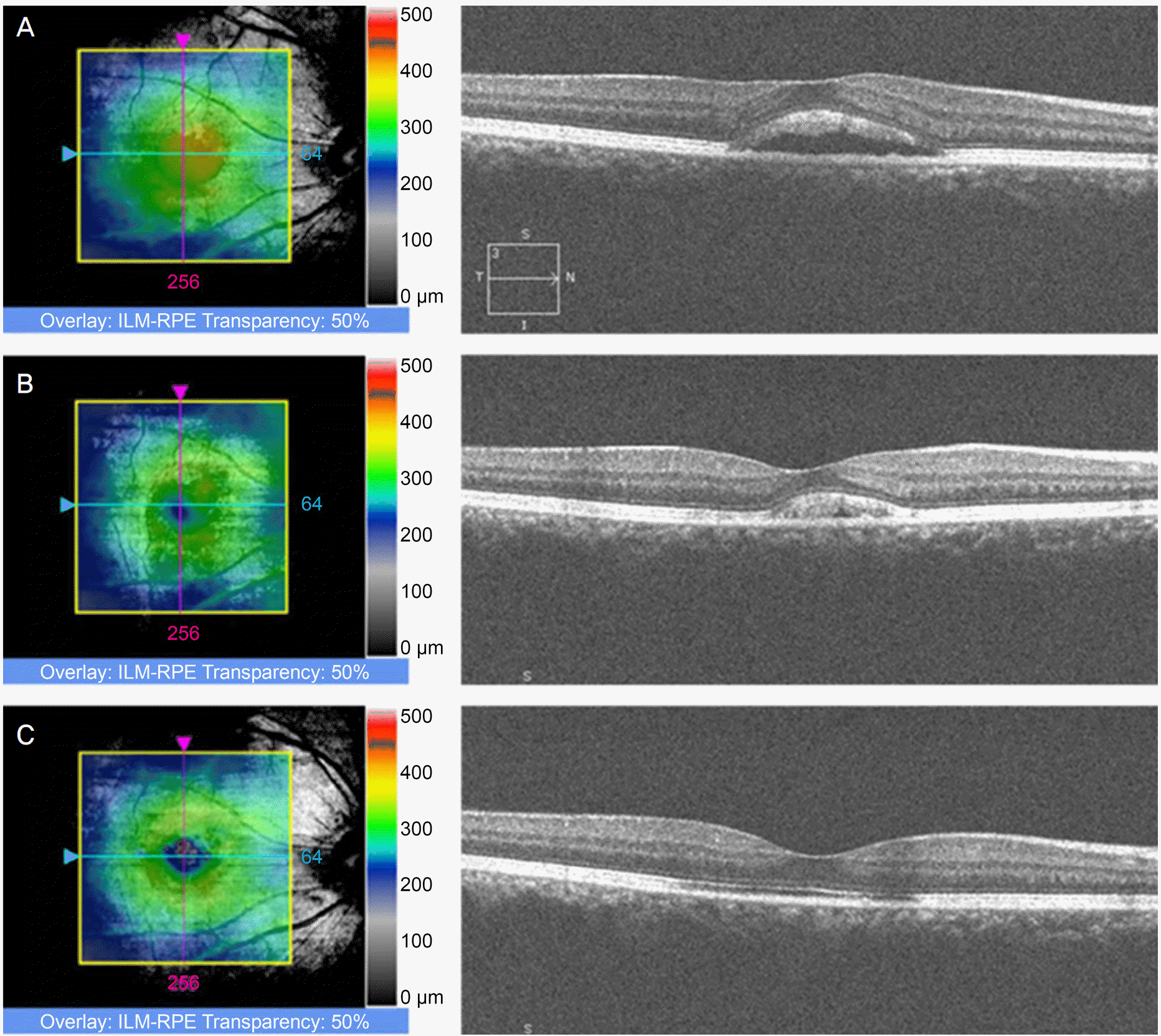 | Figure 1.Changes of macular thickness map and optical coherence tomography scan of a patient with central serous chorioretinopathy at baseline (A), at 1 month (B) and 3 months (C). The best corrected visual acuity was 0.8 at baseline and improved to 1.2 by 3 months. ILM-RPE = inner limiting membrane-retinal pigment epithelium. |
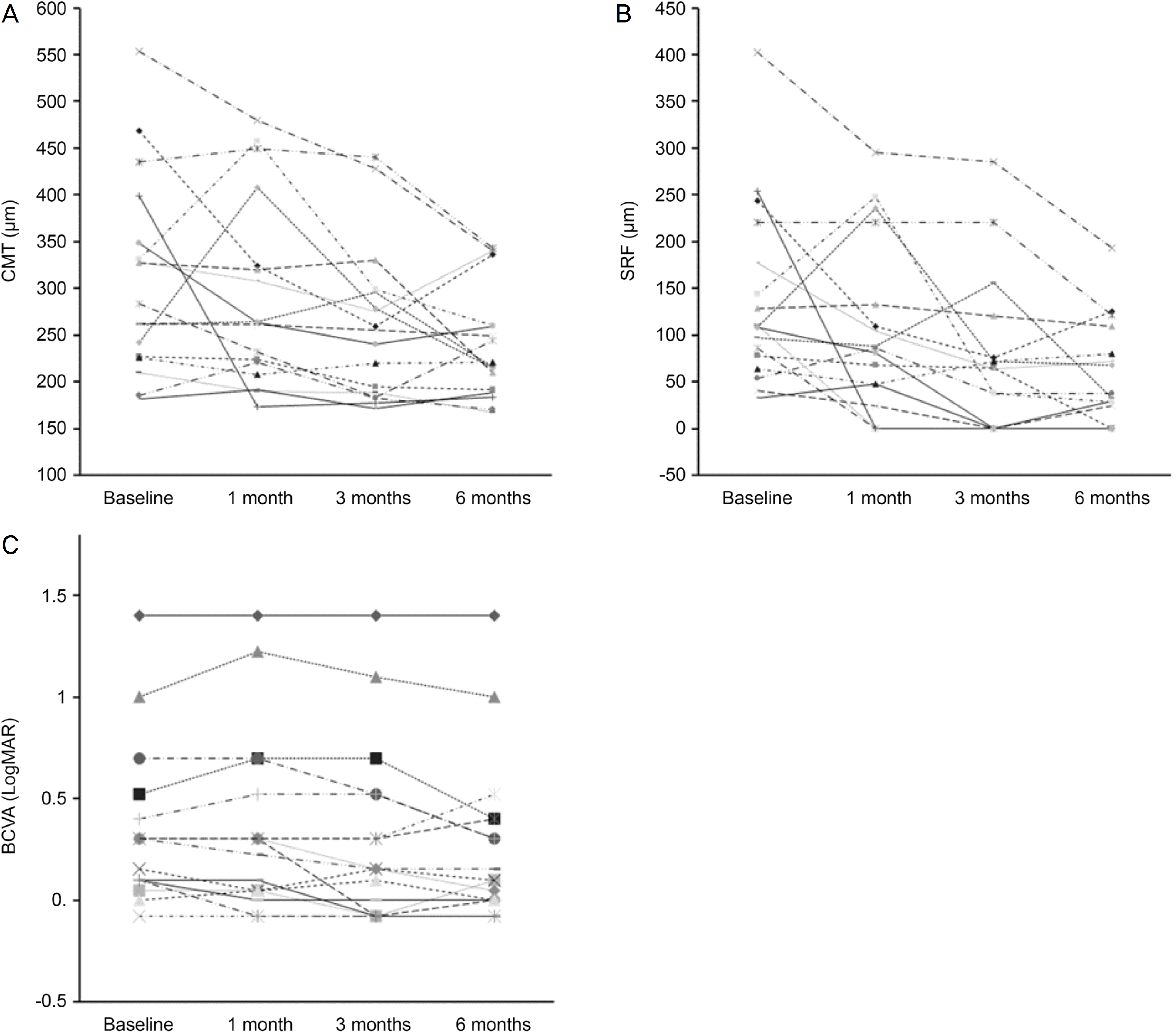 | Figure 2.SRF, CMT, BCVA changes in each spironolactone-treated eye. Individual values are presented from baseline to 6 months. CMT = central macular thickness; SRF = subretinal fluid; BCVA = best collected visual acuity; logMAR = logarithm of the minimum angle of resolution. |
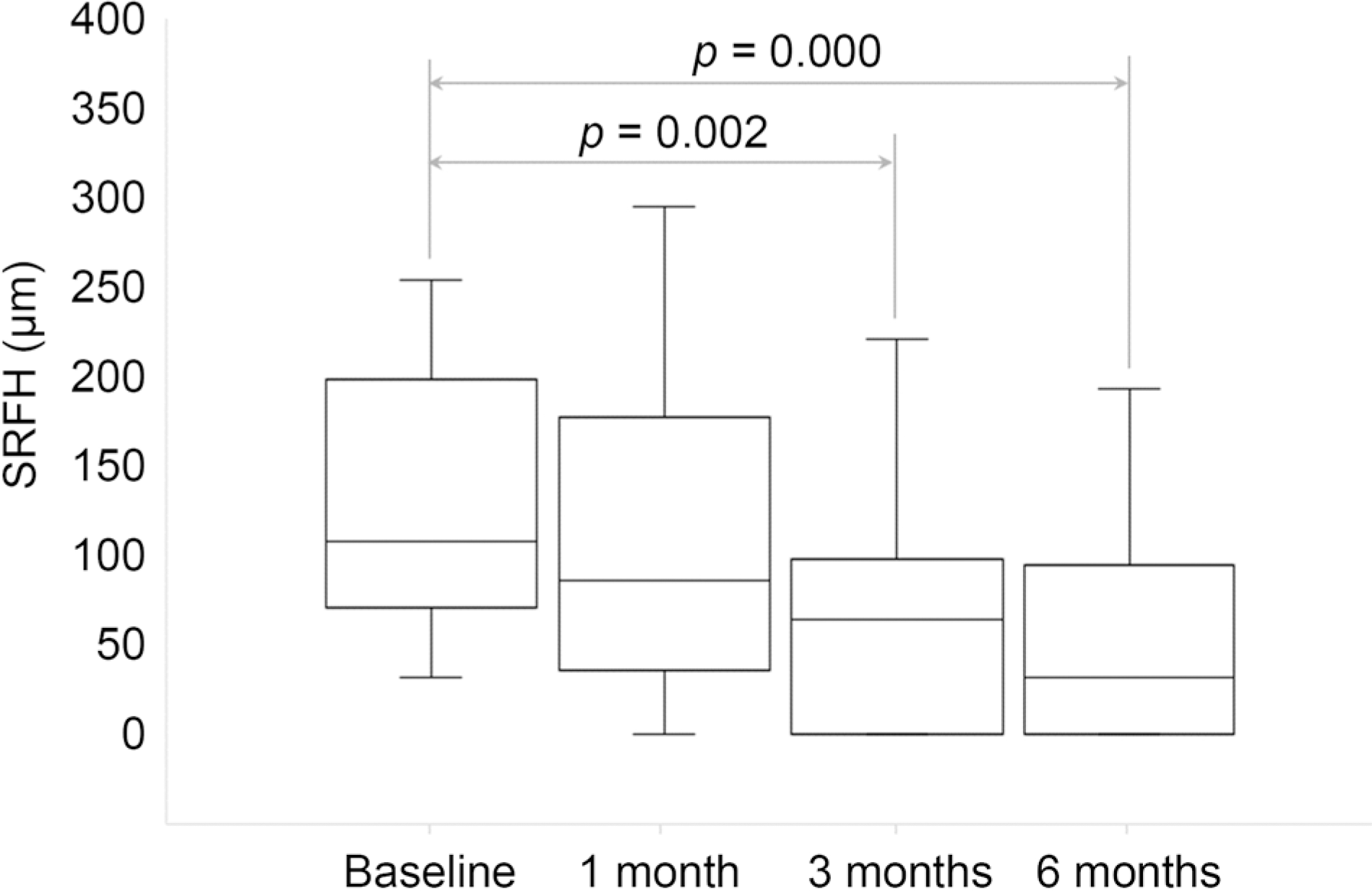 | Figure 3.Subretinal fluid height at baseline, 1 month, 3 months and 6 months after starting the treatment. The spironolactone effect was statistically significant by 3 months and 6 months (Wilcoxon signed-rank test p = 0.002, p = 0.000). SRFH = subretinal fluid height. |
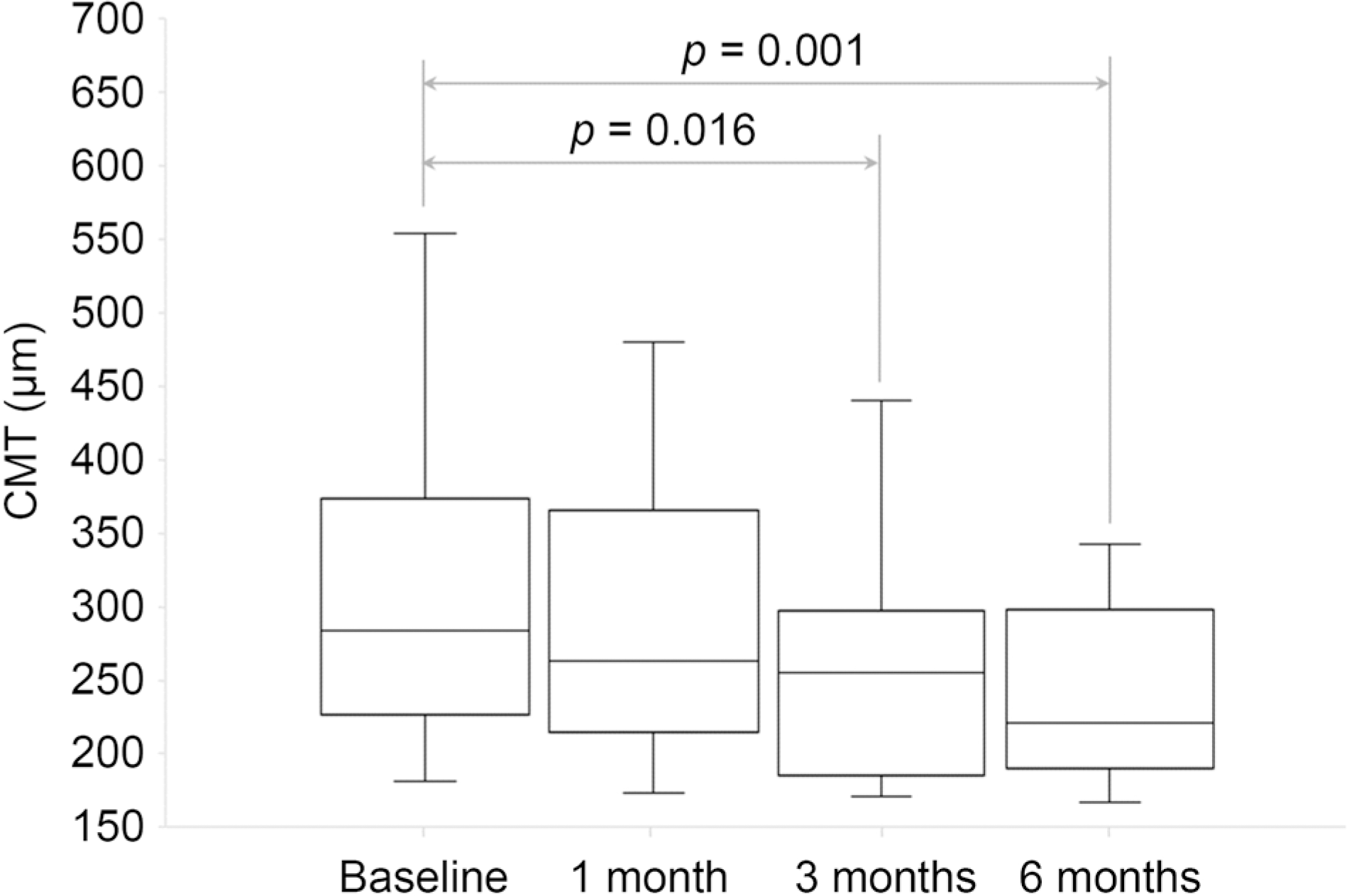 | Figure 4.Central macular thickness at baseline, 1 month, 3 months and 6 months after starting the treatment. The spironolactone effect was statistically significant by 3 months and 6 months (Wilcoxon signed-rank test p = 0.016, p = 0.001). CMT = central macular thickness. |
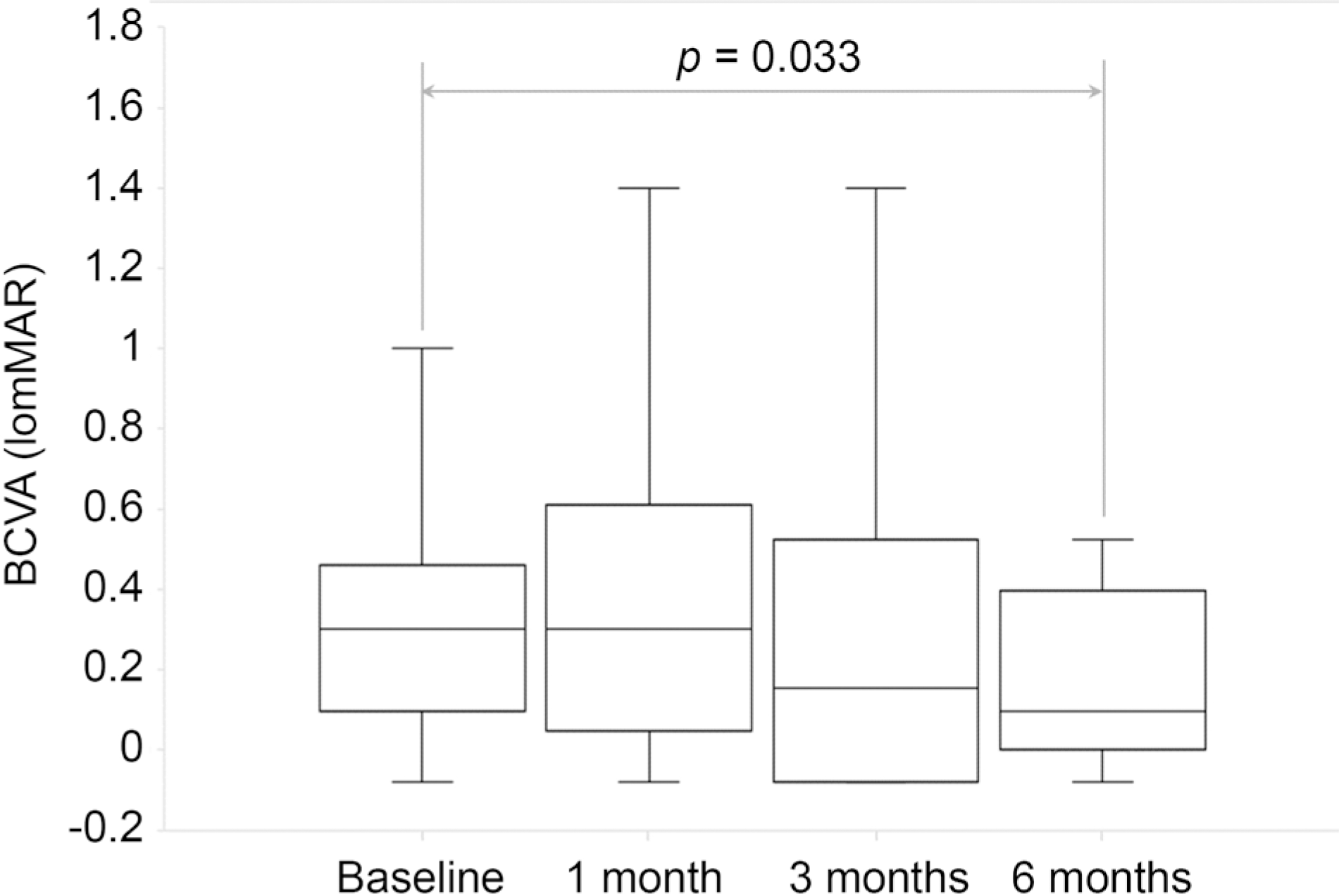 | Figure 5.Best corrected visual acuity (logMAR) at baseline, 1 month, 3 months and 6 months after starting the treatment. The spironolactone effect was statistically significant by 6 months (Wilcoxon signed-rank test, p = 0.033). BCVA = best corrected visual acuity; logMAR = logarithm of minimal angle of resolution. |
Table 1.
Characteristics of the patients
| Factor | Value (n = 17) |
|---|---|
| Number of patients (M:F) | 17 (12:5) |
| Age (years) | 56.50 ± 8.54 |
| Diabetes | 2 (0.12) |
| Hypertension | 4 (0.23) |
| Steroid use | 2 (0.12) |
Table 2.
Statistical overview of median, mean, and standard deviation of SRF, CMT, BCVA from baseline, 1 month, 3 months, and 6 months




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download