1. Laumann EO, Paik A, Rosen RC. The epidemiology of erectile dysfunction: results from the National Health and Social Life Survey. Int J Impot Res. 1999; 11 Suppl 1:S60–S64. PMID:
10554933.
2. Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000; 163:460–463. PMID:
10647654.

3. Wein AJ, Kavoussi LR, Partin AW, Peters CA. Campbell-Walsh urology. 11th ed. Philadelphia: Elsevier Saunders;2016. p. 648–649.
4. McMahon CG, Lee G, Park JK, Adaikan PG. Premature ejaculation and erectile dysfunction prevalence and attitudes in the Asia-Pacific region. J Sex Med. 2012; 9:454–465. PMID:
22023395.

5. Wein AJ, Coyne KS, Tubaro A, Sexton CC, Kopp ZS, Aiyer LP. The impact of lower urinary tract symptoms on male sexual health: EpiLUTS. BJU Int. 2009; 103 Suppl 3:33–41.

6. Gonen M, Kalkan M, Cenker A, Ozkardes H. Prevalence of premature ejaculation in Turkish men with chronic pelvic pain syndrome. J Androl. 2005; 26:601–603. PMID:
16088036.

7. Corona G, Jannini EA, Mannucci E, Fisher AD, Lotti F, Petrone L, et al. Different testosterone levels are associated with ejaculatory dysfunction. J Sex Med. 2008; 5:1991–1998. PMID:
18399946.

8. Schaeffer AJ, Datta NS, Fowler JE Jr, Krieger JN, Litwin MS, Nadler RB, et al. Overview summary statement. Diagnosis and management of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). Urology. 2002; 60(6 Suppl):1–4.
9. Tran CN, Shoskes DA. Sexual dysfunction in chronic prostatitis/chronic pelvic pain syndrome. World J Urol. 2013; 31:741–746. PMID:
23579441.

10. Pontari MA, Ruggieri MR. Mechanisms in prostatitis/chronic pelvic pain syndrome. J Urol. 2008; 179:S61–S67. PMID:
18405756.

11. Yassin AA, Nettleship JE, Almehmadi Y, Yassin DJ, El Douaihy Y, Saad F. Is there a relationship between the severity of erectile dysfunction and the comorbidity profile in men with late onset hypogonadism. Arab J Urol. 2015; 13:162–168. PMID:
26413340.

12. Li HJ, Kang DY. Prevalence of sexual dysfunction in men with chronic prostatitis/chronic pelvic pain syndrome: a meta-analysis. World J Urol. 2016; 34:1009–1017. PMID:
26546073.

13. Zhang Y, Zheng T, Tu X, Chen X, Wang Z, Chen S, et al. Erectile dysfunction in chronic prostatitis/chronic pelvic pain syndrome: outcomes from a multi-center study and risk factor analysis in a single center. PLoS One. 2016; 11:e0153054. PMID:
27120096.

14. Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000; 49:1239–1242. PMID:
11016912.

15. Kang SY, Lee JA, Sunwoo S, Yu BY, Lee JH, Cho CH, et al. Prevalence of sexual dysfunction and associated risk factors in middle-aged and elderly Korean men in primary care. J Sex Res. 2016; 53:1165–1178. PMID:
27215144.

16. Tajar A, Huhtaniemi IT, O'Neill TW, Finn JD, Pye SR, Lee DM, et al. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab. 2012; 97:1508–1516. PMID:
22419720.

17. Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014; 16:192–202. PMID:
24407185.

18. Lee JH, Lee SW. Testosterone and chronic prostatitis/chronic pelvic pain syndrome: a propensity score-matched analysis. J Sex Med. 2016; 13:1047–1055. PMID:
27235281.

19. Lee JH, Kim Y, Park YW, Lee DG. Relationship between benign prostatic hyperplasia/lower urinary tract symptoms and total serum testosterone level in healthy middle-aged eugonadal men. J Sex Med. 2014; 11:1309–1315. PMID:
24612680.

20. Tang WH, Zhuang XJ, Shu RM, Guan D, Ji YD, Zhang BL, et al. The prevalence of erectile dysfunction among subjects with late-onset hypogonadism: a population-based study in China. Int J Clin Exp Med. 2015; 8:13901–13910. PMID:
26550346.
21. Marberger M, Wilson TH, Rittmaster RS. Low serum testosterone levels are poor predictors of sexual dysfunction. BJU Int. 2011; 108:256–262. PMID:
20955266.

22. Lee JH. Associations between premature ejaculation, lower urinary tract symptoms, and erectile dysfunction in middle-aged Korean policemen. J Sex Med. 2014; 11:1512–1518. PMID:
24528521.

23. Jackson G, Betteridge J, Dean J, Eardley I, Hall R, Holdright D, et al. A systematic approach to erectile dysfunction in the cardiovascular patient: a Consensus Statement: update 2002. Int J Clin Pract. 2002; 56:663–671. PMID:
12469980.
24. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006; 295:1549–1555. PMID:
16595758.

25. Chitaley K, Kupelian V, Subak L, Wessells H. Diabetes, obesity and erectile dysfunction: field overview and research priorities. J Urol. 2009; 182:S45–S50. PMID:
19846136.

26. Corona G, Rastrelli G, Maggi M. Treatment of premature ejaculation and comorbid endocrine and metabolic disorders. In : Jannini EA, McMahon CG, Waldinger MD, editors. Premature ejaculation: from etiology to diagnosis and treatment. Milano: Springer;2013. p. 289–303.
27. Pontari MA, Ruggieri MR. Mechanisms in prostatitis/chronic pelvic pain syndrome. J Urol. 2004; 172:839–845. PMID:
15310980.

28. Schultheiss D. Urogenital infections and male sexuality: effects on ejaculation and erection. Andrologia. 2008; 40:125–129. PMID:
18336464.

29. Dimitrakov J, Joffe HV, Soldin SJ, Bolus R, Buffington CA, Nickel JC. Adrenocortical hormone abnormalities in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2008; 71:261–266. PMID:
18308097.

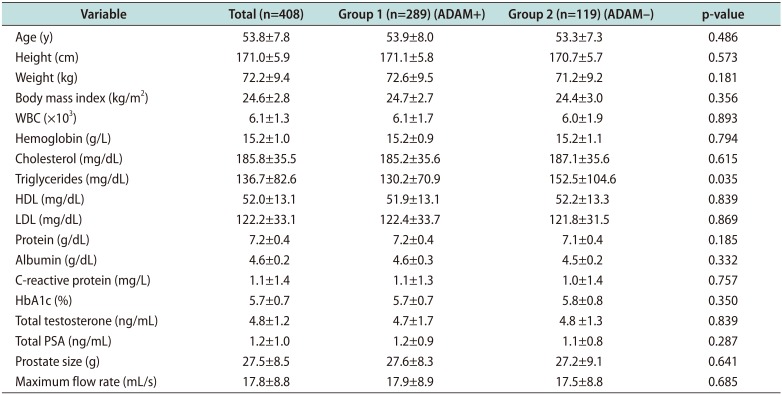
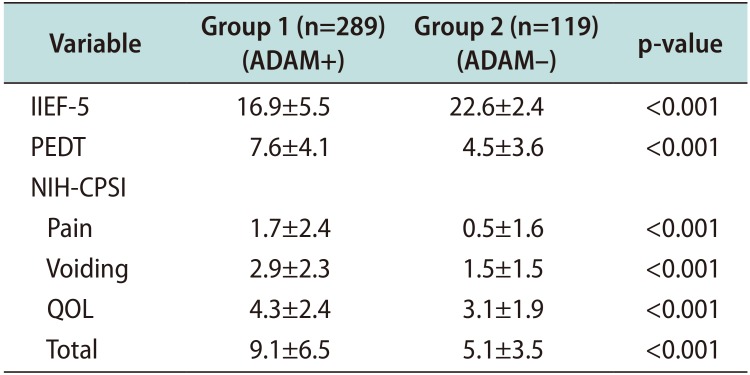
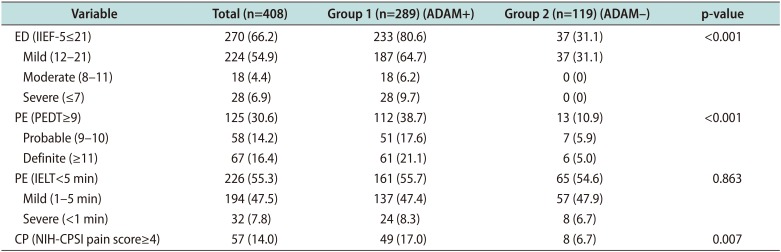
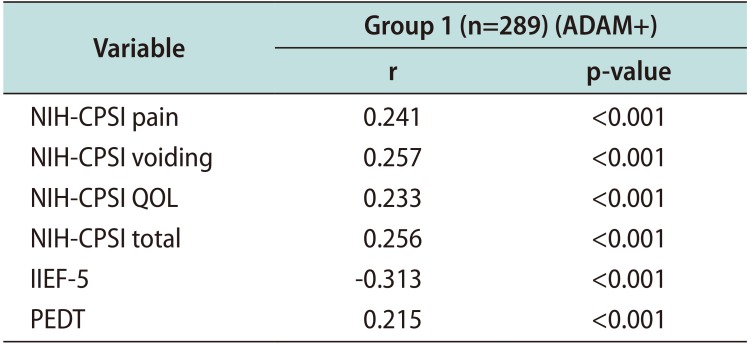




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download