Abstract
To date, the key role of vitamin D in male reproductive system has been suggested, since the expression of vitamin D receptors and metabolizing enzymes was demonstrated in the testis and spermatozoa. Nevertheless, a general consensus about the role of vitamin D in male fertility is still debated. The aim of this review is to provide an updated systematic revision of the current available literature, discussing the experimental and clinical evidence on the role of vitamin D in the regulation of testis hormone production, seminal parameters and male fertility. The consequences of vitamin D deficiency on serum levels of testicular hormones have been analysed by several observational and interventional studies, with controversial results. Equally, the experimental researches not were able to state a certain relationship between vitamin D status and testis hormone production. Possible bias, including age, body mass index, and baseline vitamin D status justified the differences among studies. As well as concerning the effect of vitamin D on semen parameters, most of the studies agreed in the possibility that vitamin D might have a positive effect on human male fertility potential, particularly through better sperm motility. Regarding pregnancy outcomes, normal level of vitamin D seems to be related to better pregnancies. However, all the previous studies displayed a wide heterogeneity in study design, population, methodology, and cut off values used for the evaluation of vitamin D status. Future studies are needed to better clarify the exact role of vitamin D on hormonal and seminal panel in both fertile and infertile men.
In recent years, vitamin D has been considered an interesting subject of study due to its pleiotropic role, including autocrine, paracrine and endocrine function on several target organs and systems. The main activity of this molecule, belonging to secosteroids group, is the regulation of calcium and phosphorus homeostasis, promoting bone mineralization [1]. The principal target organs of vitamin D are intestine, skeletal system, kidneys, and parathyroid glands [2]. Thus, vitamin D plays multiple biological effects on each one of these organs.
However, recent studies have extended the spectrum of vitamin D target organs, including the adipose tissue, thyroid, immune system, pancreas, cardiovascular system, central nervous system, and the reproductive system as well. Vitamin D deficiency (VDD) is an important risk factor not only for hyperparathyroidism, rickets, and osteomalacia, but also for further clinical conditions, such as obesity, thyroid dysfunction, autoimmune diseases, diabetes mellitus (DM), cardiovascular diseases, dementia, and cancer [3456].
The two most biologically relevant members of the vitamin D group are ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). The inactive form of vitamin D3 partially derives from dietary sources, but it is mainly synthesized by the skin with a subsequent two step activation requiring first a hepatic enzyme, resulting in 25-hydroxy-vitamin D3 which is the substrate of a renal enzyme, producing 1-α,25-dihydroxy-vitamin D3, the final active form of vitamin D3. A specific 24-hydroxylase enzyme expressed in the kidney and in different target organs is responsible for the inactivation of all forms of vitamin D3. Vitamin D plays its various biological effects through the binding and activation of vitamin D receptors (VDR).
To date, the key role of vitamin D in male reproductive system has been suggested, since the expression of VDR and vitamin D metabolizing enzymes was demonstrated in the testis and spermatozoa. Hypovitaminosis D has a negative impact on semen and hormone function, either in animals or in humans [7].
Nevertheless, a general consensus about the role of vitamin D in male fertility is still debated.
The aim of this review is to provide an updated systematic revision of the current available literature, analyzing the experimental and clinical evidence supporting the role for vitamin D in the regulation of testis hormone production, seminal parameters, and consequently male fertility.
Vitamin D3, also known as cholecalciferol, is generated by the activation of a cholesterol precursor in the skin, as the inactive form of vitamin D, due to ultraviolet B radiations [8]. Approximately, 80% to 90% of vitamin D3 derives from sunlight-induced production in the skin, while a small quantity is resultant from diet and supplements. Two distinctive enzymes play a key role in this process. Firstly, the microsomal enzyme 25-hydroxylase, located in liver, is responsible of the formation of 25-hydroxy-cholecalciferol (25-hydroxy vitamin D3), by the precursor cholecalciferol released from the skin to the blood and transported by the vitamin D-binding protein. This one is used to determine the patient's vitamin D status. Serum vitamin D levels above 30 ng/mL are considered sufficient, from 20 to 29 ng/mL insufficient and deficient under 20 ng/mL [1]. Secondly, in the kidney, the enzyme 1-α-hydroxylase leads to the formation of 1α,25-dihydroxy-cholecalciferol (1α,25-dihydroxy vitamin D3), known as the biologically active form of vitamin D [910]. Moreover, the complete series of circulating vitamin D forms is deactivated by the enzyme 24-hydroxylase, which works both in the kidney and in several target organs [11]. Biological actions of vitamin D are mediated through the VDR. The expression of VDR can be found in various organs, including bones, parathyroid glands, as well as reproductive organs [1]. Vitamin D binds to the nuclear VDR, which then heterodimerizes with the retinoid X receptor (RXR). This in turn binds to the vitamin D responsive element located in the promoter regions of the target genes [12]. This genomic pathway leading to changes in gene transcription takes hours to days [13]. A different pathway is the interaction between a cell surface receptor and second messengers, leading to a more rapid response taking seconds to minutes [113].
Mainly, the metabolism of vitamin D is regulated by parathyroid hormone (PTH), produced by parathyroid glands, and fibroblast growth factor 23 (FGF23), synthesized by osteoblasts and osteoclasts. A decreased level of circulating calcium and 25-hydroxy-vitamin D3 causes an increase of PTH secretion, that encourages 1-α-hydroxylase and inhibits 24-hydroxylase expression in the kidney, leading to a higher level of vitamin D and calcium. Otherwise, the feedback is completed when the increased levels of vitamin D and calcium suppress PTH release, blocking 1-α-hydroxylase and stimulating 24-hydroxylase [14]. Furthermore, increased levels of phosphorus and 25-hydroxy-vitamin D3, inhibit 1-α-hydroxylase and stimulate 24-hydroxylase, resulting in a reduction of vitamin D levels; to close the feedback loop, when vitamin D and phosphorus decrease, FGF23 is inhibited, generating a consequent increase in vitamin D levels [15].
The complex regulation of vitamin D metabolism is fundamental for the maintaining of a proper calciumphosphorus homeostasis. In fact, vitamin D supports calcium and phosphorus absorption in the intestine, calcium reabsorption and phosphorus excretion in the kidney, and modulates the balance between bone formation and resorption in strict dependence of the circulating calcium level [16]. In addition to bone metabolism regulation, VDR is involved in other complex functions. VDR is present in the arterial endothelium and smooth muscle cells, pancreatic B cells, monocytes, keratinocytes, and neurons [1718]. This receptor appears to be an important factor in the regulation of autoimmunity through different mechanisms: the induction of cathelicidin and the repression of interleukin-17 (IL-17), IL-2, and IL-12 expression, reducing inflammation and the risk to develop an autoimmune disease, such as type 1 DM, multiple sclerosis and rheumatoid arthritis [1819]. It seems that VDR regulates some genes expression, controlling cell differentiation and apoptosis, such as P53 and P21. Moreover, some authors showed that VDR controls glucose metabolism and can reduce the risk of cardiovascular disease by lowering homocysteine levels and inducing FOXO3, an important molecule in the prevention of oxidative stress [20]. Nevertheless, vitamin D has been recently investigated for its role in male and female fertility.
A systematic review of English-language literature was performed until December 2018 in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA statement) criteria [21]. The Medline, Scopus, Web of Science, and PubMed databases were screened separately by two different authors using a single query in order to identify all the relevant studies describing the possible association between vitamin D and male fertility. The authors screened all the articles indexed in the aforementioned databases using the following query: ((vitamin d) OR cholecalciferol) AND male fertility. Randomized clinical trials, retrospective, prospective, observational and comparative studies on humans were included, while case reports, commentaries/letters to the editor and reviews were excluded. According to the predefined inclusion and exclusion criteria, title and abstracts were screened and articles categorized. After reading the abstract, a more thorough assessment was performed by analyzing the papers full text. The references from the included studies were also searched manually to identify additional studies of interest. An excel file was built including data from the selected articles and including number of participants, interventions, comparators, outcomes, and study design, as indicated by the Systematic Review Guidance of the Centre for Reviews and Dissemination of the University of York (UK) [Centre for Reviews and Dissemination. Guidance for undertaking reviews in health care; www.york.ac.uk/crd/guidance]. The flowchart depicting the entire review process is shown in Fig. 1.
VDR and the enzymes that metabolize vitamin D are concomitantly expressed in Sertoli cells, germ cells, Leyding cells, spermatozoa and in the epithelial cells lining the male reproductive tract. The presence of vitamin D metabolizing enzymes suggests that the reproductive organs can modulate the local vitamin D response in animals and humans. The testicular somatic or germ cells seem to be able to synthesize and degrade vitamin D locally, independently from systemic vitamin D metabolism. Moreover, the VDR expression in the testis suggests that vitamin D might exert an autocrine and paracrine action, possibly displaying a role in the regulation of the testis function, therefore influencing male infertility.
The expression of VDR and vitamin D metabolizing enzymes in the male reproductive system has been widely analysed in animals and human studies. VDR protein was found in prostate, seminal vesicles, epididymis, as well as in germ cells, particularly in spermatogonia, spermatocytes and Sertoli cells [22]. The VDR protein expression was found in animal spermatozoa, but it was suppressed in the tail of epididymis [23]. In the same context, testicular testosterone synthesis enzymes appeared down-regulated in VDD diet-fed mice [24]. Otherwise, the expression of VDR protein in Leyding cells is debated in literature [25].
In human spermatozoa, by using reverse transcription polymerase chain reaction, VDR showed a heterogeneous pattern of localization, consisting in postacrosome region, neck and or mid-piece [26].
On the other hand, the expression of RXR was demonstrated in rat testis [27] in the developing or adult animals. RXRα protein was detected in Leyding cells, Sertoli cells, and germ cells. RXRβ protein was found in Sertoli cells with increasing amount during Sertoli cell maturation [2829]. RXRγ protein was discovered in Sertoli cells, Leyding cells, spermatogonia, spermatocytes, and spermatids [282930]. Unfortunately, no studies analysed the expression of RXR in humans. Concerning to the vitamin D metabolizing enzymes, few animal studies focused on this topic were found. 25-hydroxylase and 1α-hydroxylase messengers were found in animal testis, while 1α-hydroxylase protein was detected in prostate, seminal vesicles, epididymis, germ cells, Sertoli cells, and Leyding cells [233132]. Similarly, few studies were conducted in human male reproductive system. In spermatozoa, there was found 25-hydroxylase predominantly in post-acrosome region, neck and tail and 1α-hydroxylase in in post-acrosome region, neck and mid-piece. The 24-hydroxylase and 25-hydroxylase messengers and proteins were localized in prostate, seminal vesicles, epididymis and testis [26].
Some previous human observational studies, who investigated the negative effect of orchiectomy or testicular dysfunction on circulating levels of 25-hydroxyvitamin D3, proposed a possible vitamin D synthesis in the testis. An experimental study conducted in animals showed that mouse Leyding cells are able to secrete 25-hydroxy-vitamin D3, also induced by human chorionic gonadotropin (hCG) [33]. To support this theory, a subsequent study demonstrated that hCG therapy in men suffering from late-onset hypogonadism could improve significantly the levels of circulating 25-hydroxyvitamin D3 [34]. Another observational study stated the ability of germ cells to contribute to testis vitamin D synthesis, since in patients with severely damaged spermatogenesis or with a diagnosis of Sertoli-cell-only-syndrome, the level of circulating 25-hydroxy-vitamin D3 was significantly lower [35]. In this scenario, testis regulatory mechanisms could play a key role, including FGF23 and PTH-related molecules. FGF23 seems to modulate 1α-hydroxylase expression and thus vitamin D metabolism, as suggested by one study in which 1α-hydroxylase expression was significantly higher in FGF23-null mice, compared to wild-type animals [36]. To effort this theory an experimental research showed that the treatment with Kloto protein, a specific cofactor essential for the activation of FGF23, consistently inhibited 1α-hydroxylase messenger expression in mouse Sertoli cells [37]. PTH-related molecules, such as PTH-related peptide (PTH-rP) have been detected in Leyding cells and germ cells in animals [3839]. In humans these issues were not largely investigated. Indeed, FGF23 receptor expression has been found in germ cells [40], while PTH-rP was detect in Leyding cells [41], suggesting that these pathways might regulate vitamin D metabolism. In summary, the specific role of FGF23 and PTH-rP in vitamin D metabolism regulation has not been finally defined. Nevertheless, in humans, it was highlighted that circulating 25-hydroxy-vitamin D3 did not correlate with 24-hydroxylase expression in spermatozoa from healthy and infertile men [42], suggesting that locally produced vitamin D, rather than circulating vitamin D, might be involved in the local regulation of testis vitamin D metabolism.
The impact of vitamin D on male fertility proved to be dependent by the consequences on testis function. Testicular activity comprises two related processes: hormone production and spermatogenesis, which, contribute to male reproductive potential. Hormone production by the testis calls for both somatic and germ cells, and it is essential for a proper spermatogenesis [43]. The main hormones produced by the testis include testosterone, estradiol, anti-mullerian hormone (AMH), inhibin-B (INH-B), and insulin-like 3 (INSL3). Spermatogenesis process comprises spermatogonia proliferation and differentiation in to spermatocytes, spermatidogenesis, spermiogenesis, including the steps of maturation and differentiation of spermatids in mature spermatozoa, and spermiation, consisting in the release of mature spermatozoa into the lumen of the seminiferous tubules. It is widely demonstrated that paracrine and autocrine actions of intratesticular hormones play a key role in the regulation of spermatogenesis. Testosterone regulates spermatidogenesis and spermiation, performed within the testis, by actions on Sertoli cells. The precise role of estradiol in spermatogenesis is still a matter of debate, although it is well known that proper spermatogenesis requires a complex balance between testosterone and estradiol concentrations [44]. The possible role of AMH, INH-B, and INSL3 in local regulation of spermatogenesis in adult has not been yet clearly demonstrated [31].
The role of vitamin D on testosterone production has been demonstrated by in vitro, ex vivo, and in vivo studies. A first in vivo study highlighted the positive association between lower circulating testosterone levels and hypovitaminosis D in rats [45].
However, it is difficult to establish the precise mechanism in the hormonal regulation of testosterone production. The theory that, in animals, testosterone secretion might be regulated by changes in the intracellular calcium homeostasis in Leyding cells was supported by some authors. For example, osteocalcin, a hormone involved in bone metabolism and produced by osteoblasts, was suggested to be a mediator of testosterone production as a consequence of vitamin D effect [46]. About this, previous studies stated that circulating testosterone levels in a murine model were significantly decreased in the osteocalcin loss-of-function group and increased in the osteocalcin gain-of-function group [47]. Moreover, an interventional study involving vitamin D-depleted and vitamin D-replaced chickens showed that, although circulating levels of testosterone were similar among groups, the expression of calbindin-D28k, a calcium-binding protein that regulates calcium homeostasis, was significantly reduced in vitamin D-depleted animals [48]. Regarding about the role of vitamin D on estradiol production, previous in vivo studies reported that in a VDR-null mouse, both aromatase expression and activity in the testis were significantly reduced, while serum estradiol level underwent a slow decrease [49]. Moreover, others authors stated that vitamin D exerts a non-specific effect on intracellular calcium homeostasis, inducing a calcium uptake in the testis via the activation of non-genomic pathways [22]. In summary, vitamin D seems to be able to influence testosterone synthesis indirectly, by a genomic vitamin D-induced expression of osteocalcin, and directly by the expression of calbindin-D28k. Moreover, the effects on estradiol synthesis seems to be related to the direct genomic and non-genomic vitamin D-induced aromatase expression in the testis. Lastly, vitamin D does not seem to be implicated in the regulation of AMH, INH-B, and INSL3 production.
Several in vivo studies in animal models analysed the role of vitamin D status on semen quality and fertility (Table 1). One of the most outdated studies showed no effect of a VDD on the reproductive performance of cockerels in terms of body weight gains, feed intake, seminal criteria (semen volume, packed sperm volume, methylene blue reduction time), and duration of fertility and hatchability [50].
Successful mating, understood as the presence of sperm in the vaginal tract, in vitamin D deficient males was reduced by 45% when compared to vitamin D replete males. Equally, fertility and pregnancy rate resulted significantly decreased in vitamin D-depleted rats [51]. To strengthen the idea that hypovitaminosis D could be partially related to an injury of calcium homeostasis, several studies stablished the relationship between decreased calcium levels and faulty male reproductive system [52]. In this setting, vitamin D and 1,25-dihydroxycholecalciferol seemed to act on classic target tissues and regulate levels of calcium in reproductive tissues, influencing mating, fertility, and pregnancy ratio [52].
In a murine model with a targeted deletion of 25-hydroxyvitamin D 1α-hydroxylase, resulting in reduced serum calcium concentrations, it was found an increase of histological anomalies in the testis. Indeed, an hypocalcemic and hypophosphatemic male had hypergonadotropic hypogonadism, with down-regulation of testicular calcium channels, lower intracellular calcium levels, decreased sperm count and motility, and histological abnormalities of the testes. Furthermore, when serum calcium and phosphorus were normalized by the rescue diet, spermatogenesis and semen quality improved considerably [53].
Sood et al [54] investigated the impact of vitamin D depletion on the spermatogenesis process, showing lower levels of testicular glutamyl transpeptidase activity, as index of Sertoli cell function in vitamin D-deficient rats. The histological analysis of the testis in case of hypovitaminosis showed a significant decline of Leyding cells with degenerative changes in the germinal epithelium. These remarks confirmed the effect of vitamin D on testicular function, and indirectly on male infertility. A subsequent interventional study, involving the administration of three different doses of vitamin D intramuscularly, demonstrated that the injection of lower doses of vitamin D on the 120th day of age improved testicular function compared to administration of high doses [55]. This occurs as a result of the deleterious effects of hypervitaminosis D on spermatogenesis, on the basis that hypercalcemia could affect cytoplasmic activity at the mitochondrial level [56]. This finding leads to highlight the importance of an optimal dose of vitamin D in the correction of male infertility. Unfortunately, no other study has investigated what is the most appropriate dose of serum vitamin D circulating, better related to fertility panel in the normal range.
Only one study assessed the role of vitamin D in the regulation of estrogen synthesis in male gonads.
The study showed that, in a VDR null mutant male mice, sperm count and motility were decreased, while testicular histological abnormalities were also found. However, the supplementation with estradiol normalized the histological abnormalities in the male gonads, calcium supplementation increased aromatase activity and partially corrected the hypogonadism [51].
Interestingly, testicular weight and the number of spermatozoa in the cauda epididymis are significantly decreased, testicular germ cell proliferation is suppressed, and the percentage of mature seminiferous tubules is decreased in a VDD diet-fed mice [24]. Regarding reproductive outcomes, VDD had little effect on mating index, whereas fertility index was reduced in VDD-fed male offsprings. Moreover, there was a downstream trend on the numbers of implantation sites per litter when both males and females were fed with VDD diet in early life. Moreover, although there was no significant difference on the numbers of resorptions per litter and dead fetuses, the number of live fetuses was significantly reduced when both males and females were fed with VDD diet in early life [24].
To date, more recent studies demonstrated fat-by-vitamin D interaction effects on sperm motility and mitochondrial membrane potential, using the cationic reagent, JC-1 solution. However, fertilizing capacity resulted greater in animals receiving a control diet, rather than in animals receiving high-fat diet, suggesting that the high-fat diet and VDD contribute to decrease the sperm quality and consequently could decrease the fertilizing capacity [57].
Also DNA fragmentation increased significantly in the spermatozoa of animals with vitamin D deficient diet and diet-induced obesity. In doing so, the DNA damage observed could explain the low fertility potential in obese/vitamin D deficient males [58].
The consequences of VDD on serum levels of testicular hormones have been analysed by several studies, with controversial results. Furthermore, possible bias, including age, body mass index, and baseline vitamin D status justified the differences among studies. Most of the observational studies demonstrated that serum level of 25-hydroxy-vitamin D3 was not related to circulating concentrations of total or free testosterone [5960616263646566], with one study exception [67]. Furthermore, some of these studies revealed a significantly relationship between 25-hydroxyvitamin D3 and sex hormone-binding globulin (SHBG) [59606165]. In this setting, hypovitaminosis D could influence indirectly the hormonal panel, modulating the bioavailable fraction of testosterone. Otherwise, one study that evaluated the male partners from infertile couples, could not establish a relationship between circulating 25-hydroxy-vitamin D3 and, both, total and free testosterone, and SHBG [68]. Also looking for elderly patients, the results in literature are contentious. Indeed, some studies not showed a relationship between circulating 25-hydroxyvitamin D3 and total testosterone, free testosterone or SHBG [697071], while others stated a positive association with total or free testosterone [727374757677]. Furthermore, one study claimed a negative association with free testosterone [77], while two studies reported a relationship with SHBG [7377].
A recent randomized clinical trial showed that SHBG levels and testosterone/estradiol ratios were 15% and 14% lower, respectively, while free testosterone and estradiol ratios were 6 and 13% higher, respectively, in men with 25-OHD <25 nmol/L. Men with lower Ca2+ levels had a low inhibin B/FSH ratio but a lower testosterone/estradiol ratio [78].
However, the reason why there is a strong debate on this topic is probably that age-related comorbidities, including endocrinology and cardiovascular pathologies, can independently influence vitamin D assessment and circulating testosterone levels. To date, there is not sufficient data to establish a certain relationship between vitamin D status and testosterone levels.
Moreover, also the studies that focused on patients with hypogonadism have shown controversial results. Some of them reported that men with hypogonadism had significantly lower circulating 25-hydroxy-vitamin D3 compared to normogonadal men [6973], others did not find an association between hypogonadism and hypovitaminosis D [7577]. Interestingly, one study claimed a positive association between hypogonadism and higher vitamin D levels [68].
A general consensus on this subject was not even reached by the interventional studies. The results appeared extremely variable and dependent by the term of vitamin D supplementation. A very short-term (4 days) and a short-term (3 months) supplementations weren't capable to influence circulating levels of total testosterone [7980]. Otherwise, the long-term supplementation (12 months) with vitamin D2 and vitamin D3 in different-age mens' groups can generate a significant increase in total testosterone [81] or free testosterone and SHBG [82] or no changes [83]. The only study that involves a very long-term supplementation in 24 months couldn't assessed any relation [84]. The precise molecular mechanism that links vitamin D to testosterone production is still unknown. One in vitro study demonstrated that vitamin D replacement in primary culture of Leyding cells, significantly stimulated the messenger expression of enzymes involved in testosterone synthesis [85]. Moreover, some authors suggested the hypothesis that vitamin D genomic stimulation of osteocalcin expression might have a central role in regulating testosterone production by the testis [86].
Several studies in humans analysed the consequences of hypovitaminosis D on seminal panel and fertility. Most of them were clinical observational studies that assessed the correlation between vitamin D status and semen quality and/or spermatogenesis. A summary of clinical observational and interventional studies, in humans, is showed in Table 2.
However, the main findings of these studies seem controversial for some seminal parameters, although they agreed in the possibility that vitamin D might have a positive effect on human male fertility potential.
Many authors found a consistent positive association between circulating 25-hydroxy-viamin D3 levels and total sperm motility and/or progressive motility, total sperm count, and normal morphology [66878889].
Some studies showed that VDD might damage on sperm motility, suggesting that circulating 25-hydroxy-vitamin D3 has a key role in modulating sperm motility [6626678878890919293]. A recent prospective observational study showed that vitamin D deficient men had significantly lower total and progressive sperm motility and total number of motile spermatozoa, concluding that VDD and ionized calcium may influence sex steroid bioavailability and semen quality in infertile men [78].
Otherwise, the correlation between vitamin D status and sperm count and morphology is highly controversial. Indeed, vitamin D was reported to be unrelated [628990929394] or positively associated to sperm count [627887889195]. Most of the studies showed that circulating levels of 25-hydroxy-vitamin D3 were not associated with sperm morphology [6162788993949596]. Only four studies showed a positive association [66878890].
Only one study indicated that low vitamin D is not a risk factor for poor semen quality in a population of young healthy men, although a high vitamin D level was unexpectedly associated with lower crude median total sperm count and percentage of normal sperm morphology. However, after adjustment, the results led to non-significant associations [61].
Lastly, few studies included also non-obstructive azoospermic patients in their study cohort. One of them was conducted by Yang et al [66], showing that no significant differences of bone mineral density exist between infertile azoospermic men and sub-fertile patients. Otherwise, Zhu et al [91] revealed levels of serum 1,25(OH)2D3 significantly lower in azoospermic patients those than fertile men.
Furthermore, few studies analysed the reproductive outcomes in men with hypovitaminosis D. One of them stated that pregnancy and delivery rates were significantly higher in couples with normal serum levels of vitamin D rather than couples with lower levels, during cycles of timed vaginal intercourse. A trend towards a higher rate of miscarriage was observed in case of hypovitaminosis D, not reaching a statistical significance [89]. Moreover, only a study assessed vitamin D status in couples undergoing in vitro fertilization/intracytoplasmic sperm injection cycles. Among men, no correlation was found between 25(OH)D and total motility, progressive motility, count, or sperm morphology. Additionally, there was no association between 25(OH)D and ongoing pregnancy rates. Regarding joint factors in subfertility, there was no association between male 25(OH)D concentration and number of fertilized oocytes [94].
Additionally, the effects of vitamin D was also investigated in in vitro studies. Blomberg Jensen et al [90] focused on in vitro effects of intracellular calcium, sperm motility and acrosome reaction of mature spermatozoa, obtained from semen samples of 40 men from the general population. The semen samples were exposed to 1,25(OH)2D3 for 45 minutes. In this setting, 1,25(OH)2D3 increased intracellular calcium concentration in human spermatozoa through VDR-mediated calcium release from the intracellular calcium storage, increased sperm motility and induced the acrosome reaction.
One more recent in vitro study explored the relationships between both serum and seminal plasma vitamin D levels and semen quality, by incubating spermatozoa with 1,25(OH)2 vitamin D. In vitro, sperm kinetic parameters increased after incubation for 30 minutes with 1,25(OH)2 vitamin D. The upward migration of spermatozoa increased remarkably with increasing adenosine triphosphate concentration, showing a positive correlation between seminal plasma vitamin D and sperm kinetics. In this context, seminal plasma vitamin D may better reflect the status of male reproduction [95].
Only two studies were conducted in an interventional study fashion. One first interventional study analysed the effects of vitamin D3 supplementation for 3 months in men with oligo-asthenozoospermia, showing a significant increase in both sperm progressive motility and pregnancy rate, compared to not treated men [92]. Nevertheless, more recently, Blomberg Jensen et al [96] conducted a randomized clinical trial in infertile couples, whose men had shown impaired semen quality and vitamin D insufficiency (25OHD level <50 nmol/L). Infertile men were randomly assigned 1:1 to either placebo or an initial oral dose of 300,000 IU of cholecalciferol dissolved in oil, followed by receipt of tablets consisting of cholecalciferol 1,400 IU and calcium 500 mg once daily for 150 days. No differences in total sperm count or sperm concentration were found between the treatment and the placebo groups at day 150. Men treated with supplementation had lower percentage of motile spermatozoa and progressive motile spermatozoa compared with the placebo group at baseline. The percentage of spermatozoa with normal morphology was higher in the placebo group than in the treatment group. Overall, the results suggested that vitamin D supplementation was not significantly associated with changes in semen parameters. Moreover, spontaneous pregnancies tended to be higher in couples in which the man was in the treatment group. Analysing a subgroup of oligozoospermic men, vitamin D treatment increased the chance for a live birth compared with placebo [96].
However, all these previous studies displayed a wide heterogeneity in their design, especially with regard to study population, methodology and cut off values used for the evaluation of vitamin D status. Moreover, the presence of confounding factors could represent a possible bias in the interpretation of data among the studies. In this regard, the major confounders could be the high frequency of serious co-morbidities, the variation in age and use of treatment that could influence semen quality, steroidogenesis or peripheral conversion of androgens.
In conclusion, all the observational and interventional existing studies reached controversial results. Equally, the experimental researches were not able to state a certain relationship between vitamin D status and testis hormone production. As well as concerning the effects of vitamin D on semen parameters, most of these studies highlighted a potential beneficial role of vitamin D on male reproductive health, particularly through a better sperm motility. Regarding pregnancy outcomes, normal level of vitamin D seems to be related to higher pregnancy rates. Nevertheless, future studies are needed to better clarify the exact role of vitamin D on hormonal and seminal panel in both fertile and infertile men. In this setting, it becomes mandatory to establish a defined range of circulating vitamin D serum levels, better related to healthy fertility parameters and positive reproductive outcomes.
References
2. Dusilová-Sulková S. Vitamin D metabolism and vitamin D traditional and nontraditional, target organs: implications for kidney patients. J Ren Care. 2009; 35 Suppl 1:39–44. PMID: 19222730.

3. Savastano S, Barrea L, Savanelli MC, Nappi F, Di Somma C, Orio F, et al. Low vitamin D status and obesity: role of nutritionist. Rev Endocr Metab Disord. 2017; 18:215–225. PMID: 28229265.

4. Altieri B, Muscogiuri G, Barrea L, Mathieu C, Vallone CV, Mascitelli L, et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev Endocr Metab Disord. 2017; 18:335–346. PMID: 28070798.

5. Muscogiuri G, Altieri B, de Angelis C, Palomba S, Pivonello R, Colao A, et al. Shedding new light on female fertility: the role of vitamin D. Rev Endocr Metab Disord. 2017; 18:273–283. PMID: 28102491.

6. Tirabassi G, Cutini M, Muscogiuri G, Delli Muti N, Corona G, Galdiero M, et al. Association between vitamin D and sperm parameters: clinical evidence. Endocrine. 2017; 58:194–198. PMID: 27942975.

7. de Angelis C, Galdiero M, Pivonello C, Garifalos F, Menafra D, Cariati F, et al. The role of vitamin D in male fertility: a focus on the testis. Rev Endocr Metab Disord. 2017; 18:285–305. PMID: 28667465.

8. Henry HL. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab. 2011; 25:531–541. PMID: 21872796.

9. Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014; 144:132–137. PMID: 24095930.

10. Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999; 96:507–515. PMID: 10052453.

11. Veldurthy V, Wei R, Campbell M, Lupicki K, Dhawan P, Christakos S. 25-hydroxyvitamin D3 24-hydroxylase: a key regulator of 1,25(OH)2D3 catabolism and calcium homeostasis. Vitam Horm. 2016; 100:137–150. PMID: 26827951.
12. Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998; 78:1193–1231. PMID: 9790574.

13. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008; 29:726–776. PMID: 18694980.

14. Brenza HL, DeLuca HF. Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 2000; 381:143–152. PMID: 11019830.
16. DeLuca HF. The control of calcium and phosphorus metabolism by the vitamin D endocrine system. Ann N Y Acad Sci. 1980; 355:1–17. PMID: 7015957.

17. Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011; 25:543–559. PMID: 21872797.
18. Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005; 26:662–687. PMID: 15798098.

19. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006; 311:1770–1773. PMID: 16497887.

20. Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008; 8:685–698. PMID: 19172691.

21. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097. PMID: 19621072.

22. Zanatta L, Zamoner A, Gonçalves R, Zanatta AP, Bouraïma-Lelong H, Bois C, et al. Effect of 1α,25-dihydroxyvitamin D3 in plasma membrane targets in immature rat testis: ionic channels and gamma-glutamyl transpeptidase activity. Arch Biochem Biophys. 2011; 515:46–53. PMID: 21933661.

23. Mahmoudi AR, Zarnani AH, Jeddi-Tehrani M, Katouzian L, Tavakoli M, Soltanghoraei H, et al. Distribution of vitamin D receptor and 1α-hydroxylase in male mouse reproductive tract. Reprod Sci. 2013; 20:426–436. PMID: 23188491.

24. Fu L, Chen YH, Xu S, Ji YL, Zhang C, Wang H, et al. Vitamin D deficiency impairs testicular development and spermatogenesis in mice. Reprod Toxicol. 2017; 73:241–249. PMID: 28655646.

25. Merke J, Hügel U, Ritz E. Nuclear testicular 1,25-dihydroxyvitamin D3 receptors in Sertoli cells and seminiferous tubules of adult rodents. Biochem Biophys Res Commun. 1985; 127:303–309. PMID: 2983710.

26. Blomberg Jensen M, Nielsen JE, Jørgensen A, Rajpert-De Meyts E, Kristensen DM, Jørgensen N, et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010; 25:1303–1311. PMID: 20172873.

27. Thomas K, Sung DY, Chen X, Thompson W, Chen YE, McCarrey J, et al. Developmental patterns of PPAR and RXR gene expression during spermatogenesis. Front Biosci (Elite Ed). 2011; 3:1209–1220. PMID: 21622127.

28. Gaemers IC, van Pelt AM, van der Saag PT, Hoogerbrugge JW, Themmen AP, de Rooij DG. Effect of retinoid status on the messenger ribonucleic acid expression of nuclear retinoid receptors alpha, beta, and gamma, and retinoid X receptors alpha, beta, and gamma in the mouse testis. Endocrinology. 1997; 138:1544–1551. PMID: 9075714.
29. Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, et al. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006; 147:96–110. PMID: 16210368.

30. Dufour JM, Kim KH. Cellular and subcellular localization of six retinoid receptors in rat testis during postnatal development: identification of potential heterodimeric receptors. Biol Reprod. 1999; 61:1300–1308. PMID: 10529278.
31. Blomberg Jensen M, Lieben L, Nielsen JE, Willems A, Jørgensen A, Juul A, et al. Characterization of the testicular, epididymal and endocrine phenotypes in the Leuven Vdrdeficient mouse model: targeting estrogen signalling. Mol Cell Endocrinol. 2013; 377:93–102. PMID: 23850520.

32. Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Expression patterns of mouse and human CYP orthologs (families 1–4) during development and in different adult tissues. Arch Biochem Biophys. 2005; 436:50–61. PMID: 15752708.

33. De Toni L, De Filippis V, Tescari S, Ferigo M, Ferlin A, Scattolini V, et al. Uncarboxylated osteocalcin stimulates 25-hydroxy vitamin D production in Leydig cell line through a GPRC6a-dependent pathway. Endocrinology. 2014; 155:4266–4274. PMID: 25093461.

34. La Vignera S, Condorelli RA, Cimino L, Russo GI, Morgia G, Calogero AE. Late-onset hypogonadism: the advantages of treatment with human chorionic gonadotropin rather than testosterone. Aging Male. 2016; 19:34–39. PMID: 26488941.

35. Foresta C, Strapazzon G, De Toni L, Perilli L, Di Mambro A, Muciaccia B, et al. Bone mineral density and testicular failure: evidence for a role of vitamin D 25-hydroxylase in human testis. J Clin Endocrinol Metab. 2011; 96:E646–E652. PMID: 21270327.

36. Schepisi G, De Padova S, Scarpi E, Lolli C, Gurioli G, Menna C, et al. Vitamin D status among long-term survivors of testicular cancer. Oncotarget. 2017; 8:36780–36786. PMID: 28030821.

37. Imai M, Ishikawa K, Matsukawa N, Kida I, Ohta J, Ikushima M, et al. Klotho protein activates the PKC pathway in the kidney and testis and suppresses 25-hydroxyvitamin D3 1alphahydroxylase gene expression. Endocrine. 2004; 25:229–234. PMID: 15758250.
38. Usdin TB, Paciga M, Riordan T, Kuo J, Parmelee A, Petukova G, et al. Tuberoinfundibular Peptide of 39 residues is required for germ cell development. Endocrinology. 2008; 149:4292–4300. PMID: 18483145.

39. Usdin TB, Bonner TI, Harta G, Mezey E. Distribution of parathyroid hormone-2 receptor messenger ribonucleic acid in rat. Endocrinology. 1996; 137:4285–4297. PMID: 8828488.

40. Steger K, Tetens F, Seitz J, Grothe C, Bergmann M. Localization of fibroblast growth factor 2 (FGF-2) protein and the receptors FGFR 1-4 in normal human seminiferous epithelium. Histochem Cell Biol. 1998; 110:57–62. PMID: 9681690.

41. Asa SL, Henderson J, Goltzman D, Drucker DJ. Parathyroid hormone-like peptide in normal and neoplastic human endocrine tissues. J Clin Endocrinol Metab. 1990; 71:1112–1118. PMID: 2229275.

42. Blomberg Jensen M, Jørgensen A, Nielsen JE, Bjerrum PJ, Skalkam M, Petersen JH, et al. Expression of the vitamin D metabolizing enzyme CYP24A1 at the annulus of human spermatozoa may serve as a novel marker of semen quality. Int J Androl. 2012; 35:499–510. PMID: 22404291.

43. O'Donnell L, Stanton P, de Kretser DM. Endocrinology of the male reproductive system and spermatogenesis. In : Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, editors. Endotext. South Dartmouth: MDText.com, Inc.;2000.
44. O'Shaughnessy PJ. Hormonal control of germ cell development and spermatogenesis. Semin Cell Dev Biol. 2014; 29:55–65. PMID: 24598767.
45. Sonnenberg J, Luine VN, Krey LC, Christakos S. 1,25-Dihydroxyvitamin D3 treatment results in increased choline acetyltransferase activity in specific brain nuclei. Endocrinology. 1986; 118:1433–1439. PMID: 3753932.
46. Zanatta L, Zamoner A, Zanatta AP, Bouraïma-Lelong H, Delalande C, Bois C, et al. Nongenomic and genomic effects of 1α,25(OH)2 vitamin D3 in rat testis. Life Sci. 2011; 89:515–523. PMID: 21565203.

47. Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011; 144:796–809. PMID: 21333348.

48. Inpanbutr N, Reiswig JD, Bacon WL, Slemons RD, Iacopino AM. Effect of vitamin D on testicular CaBP28K expression and serum testosterone in chickens. Biol Reprod. 1996; 54:242–248. PMID: 8838022.

49. Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000; 141:1317–1324. PMID: 10746634.

50. Lillie RJ. Inefficacy of dietary deficiencies of vitamins A, D3 and riboflavin on the reproductive performance of mature cockerels. Poult Sci. 1973; 52:1629–1636. PMID: 4359251.

51. Kwiecinski GG, Petrie GI, DeLuca HF. Vitamin D is necessary for reproductive functions of the male rat. J Nutr. 1989; 119:741–744. PMID: 2723823.

52. Uhland AM, Kwiecinski GG, DeLuca HF. Normalization of serum calcium restores fertility in vitamin D-deficient male rats. J Nutr. 1992; 122:1338–1344. PMID: 1588451.

53. Sun W, Chen L, Zhang W, Wang R, Goltzman D, Miao D. Active vitamin D deficiency mediated by extracellular calcium and phosphorus results in male infertility in young mice. Am J Physiol Endocrinol Metab. 2015; 308:E51–E62. PMID: 25370849.

54. Sood S, Marya RK, Reghunandanan R, Singh GP, Jaswal TS, Gopinathan K. Effect of vitamin D deficiency on testicular function in the rat. Ann Nutr Metab. 1992; 36:203–208. PMID: 1471857.

55. Sood S, Reghunandanan R, Reghunandanan V, Marya RK, Singh PI. Effect of vitamin D repletion on testicular function in vitamin D-deficient rats. Ann Nutr Metab. 1995; 39:95–98. PMID: 7625775.
56. Scarpelli DG, Tremblay G, Pearse AG. A comparative cytochemical and cytologic study of vitamin D induced nephrocalcinosis. Am J Pathol. 1960; 36:331–353. PMID: 14442251.
57. Merino O, Sánchez R, Gregorio MB, Sampaio F, Risopatrón J. Effect of high-fat and vitamin D deficient diet on rat sperm quality and fertility. Theriogenology. 2019; 125:6–11. PMID: 30368129.

58. Merino O, Sánchez R, Gregorio BM, Sampaio FJ, Risopatrón J. Effects of diet-induced obesity and deficient in vitamin D on spermatozoa function and DNA integrity in sprague-dawley rats. Biomed Res Int. 2018; 2018:5479057. PMID: 30596095.

59. Chin KY, Ima-Nirwana S, Wan Ngah WZ. Vitamin D is significantly associated with total testosterone and sex hormone-binding globulin in Malaysian men. Aging Male. 2015; 18:175–179. PMID: 26004987.

60. Välimäki VV, Alfthan H, Ivaska KK, Löyttyniemi E, Pettersson K, Stenman UH, et al. Serum estradiol, testosterone, and sex hormone-binding globulin as regulators of peak bone mass and bone turnover rate in young Finnish men. J Clin Endocrinol Metab. 2004; 89:3785–3789. PMID: 15292305.
61. Ramlau-Hansen CH, Moeller UK, Bonde JP, Olsen J, Thulstrup AM. Are serum levels of vitamin D associated with semen quality? Results from a cross-sectional study in young healthy men. Fertil Steril. 2011; 95:1000–1004. PMID: 21122842.

62. Hammoud AO, Meikle AW, Peterson CM, Stanford J, Gibson M, Carrell DT. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J Androl. 2012; 14:855–859. PMID: 23042450.

63. Livshits G, Karasik D, Seibel MJ. Statistical genetic analysis of plasma levels of vitamin D: familial study. Ann Hum Genet. 1999; 63:429–439. PMID: 10735584.

64. Wulaningsih W, Van Hemelrijck M, Michaelsson K, Kanarek N, Nelson WG, Ix JH, et al. Association of serum inorganic phosphate with sex steroid hormones and vitamin D in a nationally representative sample of men. Andrology. 2014; 2:967–976. PMID: 25270590.

65. Ceglia L, Chiu GR, Harris SS, Araujo AB. Serum 25-hydroxyvitamin D concentration and physical function in adult men. Clin Endocrinol (Oxf). 2011; 74:370–376. PMID: 21083597.

66. Yang B, Sun H, Wan Y, Wang H, Qin W, Yang L, et al. Associations between testosterone, bone mineral density, vitamin D and semen quality in fertile and infertile Chinese men. Int J Androl. 2012; 35:783–792. PMID: 22713128.

67. Anic GM, Albanes D, Rohrmann S, Kanarek N, Nelson WG, Bradwin G, et al. Association between serum 25-hydroxyvitamin D and serum sex steroid hormones among men in NHANES. Clin Endocrinol (Oxf). 2016; 85:258–266. PMID: 26991691.

68. Lerchbaum E, Pilz S, Trummer C, Rabe T, Schenk M, Heijboer AC, et al. Serum vitamin D levels and hypogonadism in men. Andrology. 2014; 2:748–754. PMID: 25044703.

69. Wang N, Han B, Li Q, Chen Y, Chen Y, Xia F, et al. Vitamin D is associated with testosterone and hypogonadism in Chinese men: results from a cross-sectional SPECT-China study. Reprod Biol Endocrinol. 2015; 13:74. PMID: 26177638.

70. Lee DM, Tajar A, Pye SR, Boonen S, Vanderschueren D, Bouillon R, et al. Association of hypogonadism with vitamin D status: the European Male Ageing Study. Eur J Endocrinol. 2012; 166:77–85. PMID: 22048968.

71. Chen RY, Nordin BE, Need AG, Scopacasa F, Wishart J, Morris HA, et al. Relationship between calcium absorption and plasma dehydroepiandrosterone sulphate (DHEAS) in healthy males. Clin Endocrinol (Oxf). 2008; 69:864–869. PMID: 18419789.

72. Nimptsch K, Platz EA, Willett WC, Giovannucci E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin Endocrinol (Oxf). 2012; 77:106–112. PMID: 22220644.

73. Wehr E, Pilz S, Boehm BO, März W, Obermayer-Pietsch B. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol (Oxf). 2010; 73:243–248. PMID: 20050857.

74. Tak YJ, Lee JG, Kim YJ, Park NC, Kim SS, Lee S, et al. Serum 25-hydroxyvitamin D levels and testosterone deficiency in middle-aged Korean men: a cross-sectional study. Asian J Androl. 2015; 17:324–328. PMID: 25532570.

75. Rafiq R, van Schoor NM, Sohl E, Zillikens MC, Oosterwerff MM, Schaap L, et al. Associations of vitamin D status and vitamin D-related polymorphisms with sex hormones in older men. J Steroid Biochem Mol Biol. 2016; 164:11–17. PMID: 26610790.

76. Heijboer AC, Oosterwerff M, Schroten NF, Eekhoff EM, Chel VG, de Boer RA, et al. Vitamin D supplementation and testosterone concentrations in male human subjects. Clin Endocrinol (Oxf). 2015; 83:105–110. PMID: 25557316.

77. Zhao D, Ouyang P, de Boer IH, Lutsey PL, Farag YM, Guallar E, et al. Serum vitamin D and sex hormones levels in men and women: the Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas. 2017; 96:95–102. PMID: 28041602.

78. Blomberg Jensen M, Gerner Lawaetz J, Andersson AM, Petersen JH, Nordkap L, Bang AK, et al. Vitamin D deficiency and low ionized calcium are linked with semen quality and sex steroid levels in infertile men. Hum Reprod. 2016; 31:1875–1885. PMID: 27496946.

79. Zofková I, Scholz G, Stárka L. Effect of calcitonin and 1,25(OH)2-vitamin D3 on the FSH, LH and testosterone secretion at rest and LHRH stimulated secretion. Horm Metab Res. 1989; 21:682–685. PMID: 2515139.
80. Foresta C, Calogero AE, Lombardo F, Lenzi A, Ferlin A. Lateonset hypogonadism: beyond testosterone. Asian J Androl. 2015; 17:236–238. PMID: 25248651.

81. Canguven O, Talib RA, El Ansari W, Yassin DJ, Al Naimi A. Vitamin D treatment improves levels of sexual hormones, metabolic parameters and erectile function in middle-aged vitamin D deficient men. Aging Male. 2017; 20:9–16. PMID: 28074679.

82. Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, et al. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011; 43:223–225. PMID: 21154195.

83. Jorde R, Grimnes G, Hutchinson MS, Kjærgaard M, Kamycheva E, Svartberg J. Supplementation with vitamin D does not increase serum testosterone levels in healthy males. Horm Metab Res. 2013; 45:675–681. PMID: 23686706.

84. Ferlin A, Selice R, Di Mambro A, Ghezzi M, Di Nisio A, Caretta N, et al. Role of vitamin D levels and vitamin D supplementation on bone mineral density in Klinefelter syndrome. Osteoporos Int. 2015; 26:2193–2202. PMID: 25963234.

85. Hofer D, Münzker J, Schwetz V, Ulbing M, Hutz K, Stiegler P, et al. Testicular synthesis and vitamin D action. J Clin Endocrinol Metab. 2014; 99:3766–3773. PMID: 24937537.

86. Oury F, Ferron M, Huizhen W, Confavreux C, Xu L, Lacombe J, et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest. 2013; 123:2421–2433. PMID: 23728177.

87. Rehman R, Lalani S, Baig M, Nizami I, Rana Z, Gazzaz ZJ. Association between vitamin D, reproductive hormones and sperm parameters in infertile male subjects. Front Endocrinol (Lausanne). 2018; 9:607. PMID: 30386296.

88. Akhavizadegan H, Karbakhsh M. Comparison of serum vitamin D between fertile and infertile men in a vitamin D deficient endemic area: a case-control study. Urologia. 2017; 84:218–220. PMID: 28665459.

89. Tartagni M, Matteo M, Baldini D, Tartagni MV, Alrasheed H, De Salvia MA, et al. Males with low serum levels of vitamin D have lower pregnancy rates when ovulation induction and timed intercourse are used as a treatment for infertile couples: results from a pilot study. Reprod Biol Endocrinol. 2015; 13:127. PMID: 26589555.

90. Blomberg Jensen M, Bjerrum PJ, Jessen TE, Nielsen JE, Joensen UN, Olesen IA, et al. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod. 2011; 26:1307–1317. PMID: 21427118.

91. Zhu CL, Xu QF, Li SX, Wei YC, Zhu GC, Yang C, et al. Investigation of serum vitamin D levels in Chinese infertile men. Andrologia. 2016; 48:1261–1266. PMID: 26992658.

92. Deng X, Song Y, Manson JE, Signorello LB, Zhang SM, Shrubsole MJ, et al. Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013; 11:187. PMID: 23981518.

93. Abbasihormozi S, Kouhkan A, Alizadeh AR, Shahverdi AH, Nasr-Esfahani MH, Sadighi Gilani MA, et al. Association of vitamin D status with semen quality and reproductive hormones in Iranian subfertile men. Andrology. 2017; 5:113–118. PMID: 27792863.

94. Neville G, Martyn F, Kilbane M, O'Riordan M, Wingfield M, McKenna M, et al. Vitamin D status and fertility outcomes during winter among couples undergoing in vitro fertilization/intracytoplasmic sperm injection. Int J Gynaecol Obstet. 2016; 135:172–176. PMID: 27530219.

95. Jueraitetibaike K, Ding Z, Wang DD, Peng LP, Jing J, Chen L, et al. The effect of vitamin D on sperm motility and the underlying mechanism. Asian J Androl. 2019; DOI: 10.4103/aja.aja_105_18. [Epub].

96. Blomberg Jensen M, Lawaetz JG, Petersen JH, Juul A, Jørgensen N. Effects of vitamin D supplementation on semen quality, reproductive hormones, and live birth rate: a randomized clinical trial. J Clin Endocrinol Metab. 2018; 103:870–881. PMID: 29126319.

Table 1
Effects on seminal parameters in animals
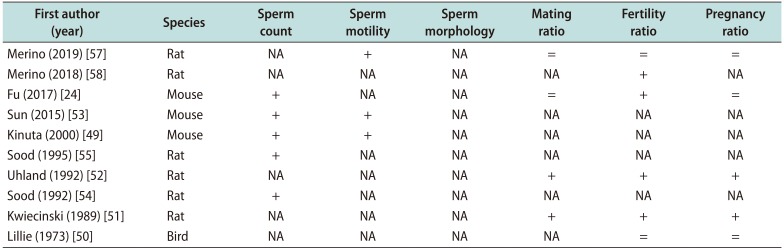
| First author (year) | Species | Sperm count | Sperm motility | Sperm morphology | Mating ratio | Fertility ratio | Pregnancy ratio |
|---|---|---|---|---|---|---|---|
| Merino (2019) [57] | Rat | NA | + | NA | = | = | = |
| Merino (2018) [58] | Rat | NA | NA | NA | NA | + | NA |
| Fu (2017) [24] | Mouse | + | NA | NA | = | + | = |
| Sun (2015) [53] | Mouse | + | + | NA | NA | NA | NA |
| Kinuta (2000) [49] | Mouse | + | + | NA | NA | NA | NA |
| Sood (1995) [55] | Rat | + | NA | NA | NA | NA | NA |
| Uhland (1992) [52] | Rat | NA | NA | NA | + | + | + |
| Sood (1992) [54] | Rat | + | NA | NA | NA | NA | NA |
| Kwiecinski (1989) [51] | Rat | NA | NA | NA | + | + | + |
| Lillie (1973) [50] | Bird | NA | NA | NA | NA | = | = |
Table 2
Effects on seminal parameters in humans
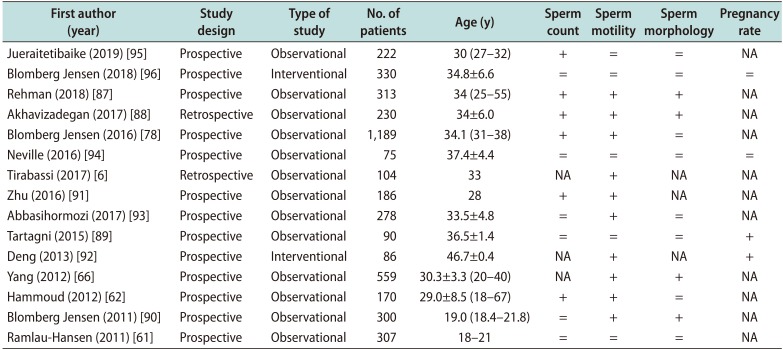
| First author (year) | Study design | Type of study | No. of patients | Age (y) | Sperm count | Sperm motility | Sperm morphology | Pregnancy rate |
|---|---|---|---|---|---|---|---|---|
| Jueraitetibaike (2019) [95] | Prospective | Observational | 22 | 30 (27–32) | + | = | = | NA |
| Blomberg Jensen (2018) [96] | Prospective | Interventional | 330 | 34.8±6.6 | = | = | = | = |
| Rehman (2018) [87] | Prospective | Observational | 313 | 34 (25–55) | + | + | + | NA |
| Akhavizadegan (2017) [88] | Retrospective | Observational | 230 | 34±6.0 | + | + | + | NA |
| Blomberg Jensen (2016) [78] | Prospective | Observational | 1,189 | 34.1 (31–38) | + | + | = | NA |
| Neville (2016) [94] | Prospective | Observational | 75 | 37.4±4.4 | = | = | = | = |
| Tirabassi (2017) [6] | Retrospective | Observational | 104 | 33 | NA | + | NA | NA |
| Zhu (2016) [91] | Prospective | Observational | 186 | 28 | + | + | NA | NA |
| Abbasihormozi (2017) [93] | Prospective | Observational | 278 | 33.5±4.8 | = | + | = | NA |
| Tartagni (2015) [89] | Prospective | Observational | 90 | 36.5±1.4 | = | = | = | + |
| Deng (2013) [92] | Prospective | Interventional | 86 | 46.7±0.4 | NA | + | NA | + |
| Yang (2012) [66] | Prospective | Observational | 559 | 30.3±3.3 (20–40) | NA | + | + | NA |
| Hammoud (2012) [62] | Prospective | Observational | 170 | 29.0±8.5 (18–67) | + | + | = | NA |
| Blomberg Jensen (2011) [90] | Prospective | Observational | 300 | 19.0 (18.4–21.8) | = | + | + | NA |
| Ramlau-Hansen (2011) [61] | Prospective | Observational | 307 | 18–21 | = | = | = | NA |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download