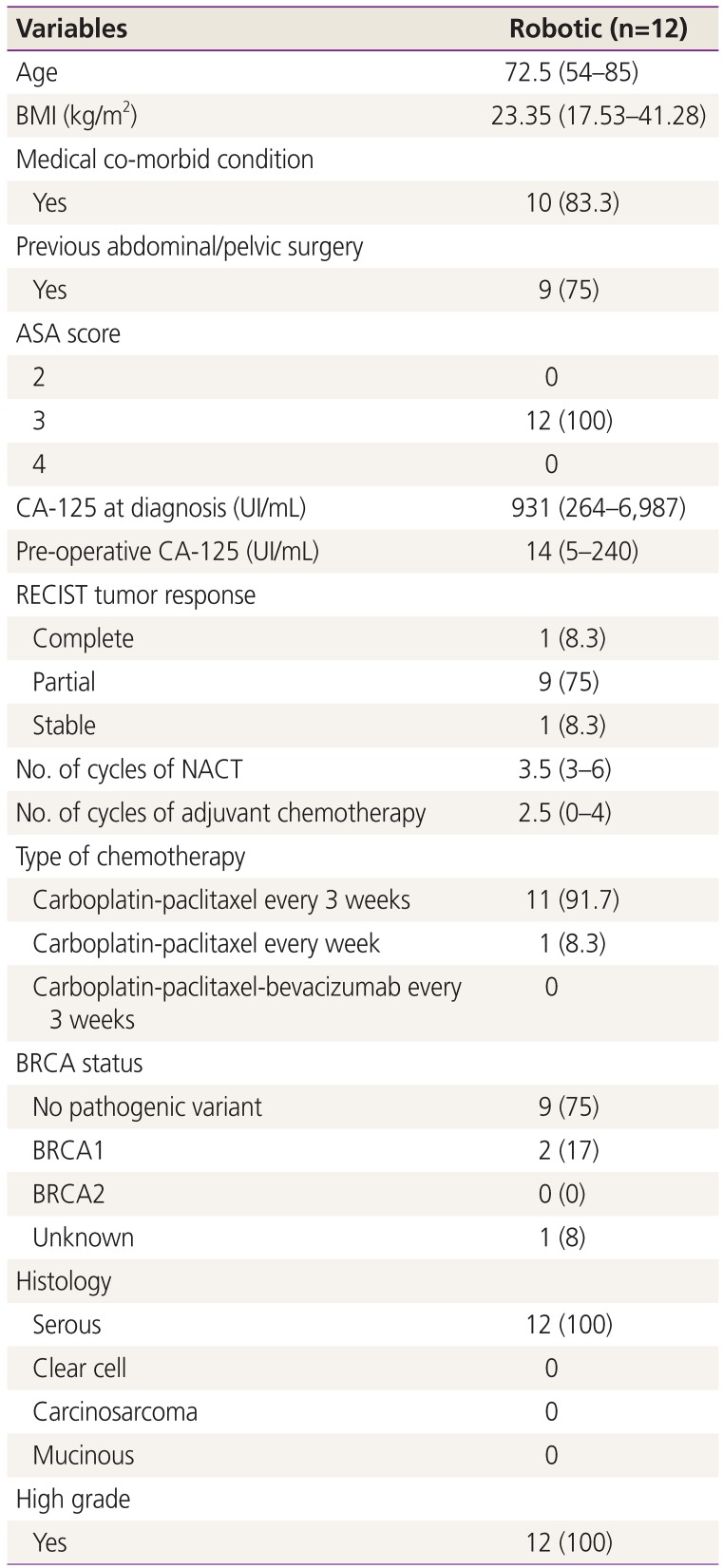
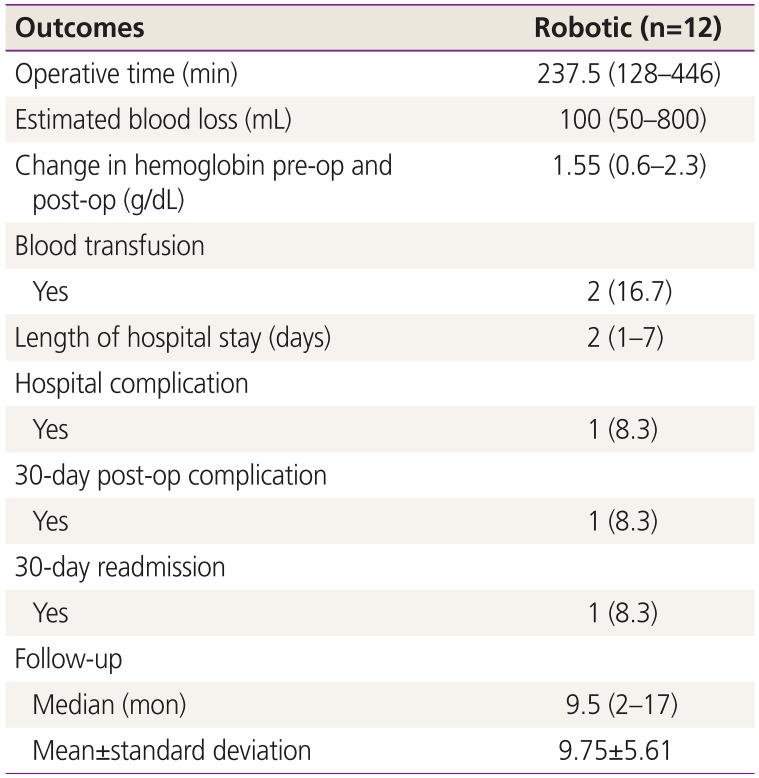
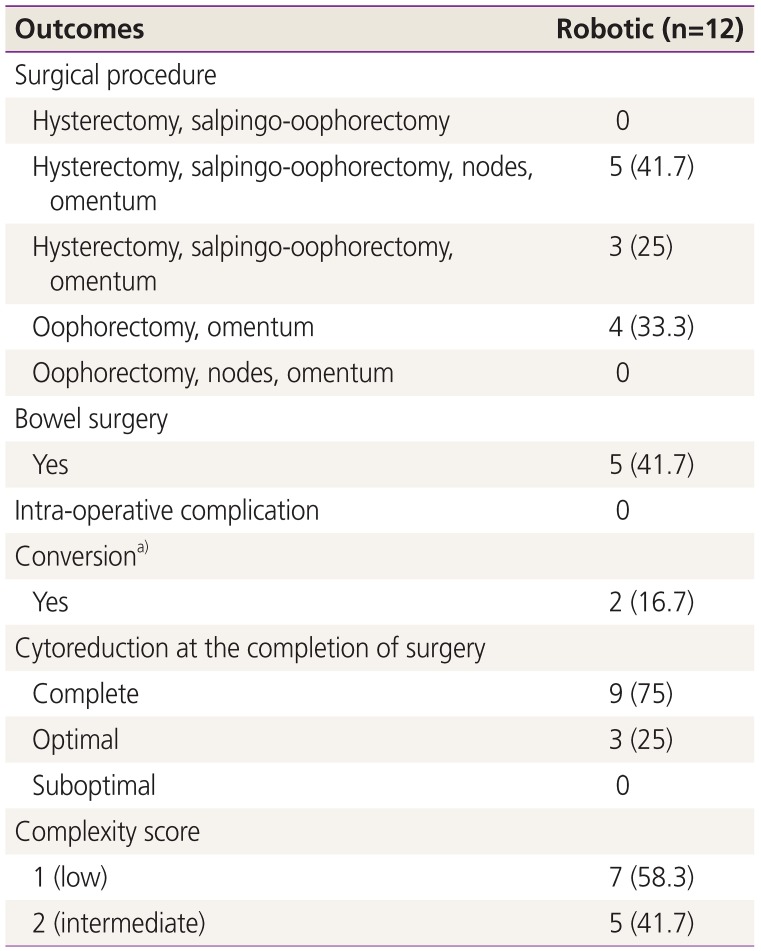
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34. PMID:
30620402.

2. Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010; 363:943–953. PMID:
20818904.

3. Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015; 386:249–257. PMID:
26002111.

4. Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Nakamura K, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer. 2016; 64:22–31. PMID:
27323348.

5. Vergote I, Coens C, Nankivell M, Kristensen GB, Parmar MK, Ehlen T, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018; 19:1680–1687. PMID:
30413383.

6. Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016; 34:3460–3473. PMID:
27502591.

7. Chang SJ, Bristow RE. Evolution of surgical treatment paradigms for advanced-stage ovarian cancer: redefining ‘optimal’ residual disease. Gynecol Oncol. 2012; 125:483–492. PMID:
22366151.

8. Hamilton CA, Miller A, Casablanca Y, Horowitz NS, Rungruang B, Krivak TC, et al. Clinicopathologic characteristics associated with long-term survival in advanced epithelial ovarian cancer: an NRG Oncology/Gynecologic Oncology Group ancillary data study. Gynecol Oncol. 2018; 148:275–280. PMID:
29195926.

9. Schorge JO, Bregar AJ, Durfee J, Berkowitz RS. Meigs to modern times: the evolution of debulking surgery in advanced ovarian cancer. Gynecol Oncol. 2018; 149:447–454. PMID:
29525276.

10. Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): final analysis of peri-operative outcome. Eur J Cancer. 2016; 59:22–33. PMID:
26998845.

11. Melamed A, Nitecki R, Boruta DM 2nd, Del Carmen MG, Clark RM, Growdon WB, et al. Laparoscopy compared with laparotomy for debulking ovarian cancer after neoadjuvant chemotherapy. Obstet Gynecol. 2017; 129:861–869. PMID:
28383367.

12. Gueli Alletti S, Bottoni C, Fanfani F, Gallotta V, Chiantera V, Costantini B, et al. Minimally invasive interval debulking surgery in ovarian neoplasm (MISSION trial-NCT02324595): a feasibility study. Am J Obstet Gynecol. 2016; 214:503.e1–503.e6. PMID:
26529370.

14. Ergina PL, Cook JA, Blazeby JM, Boutron I, Clavien PA, Reeves BC, et al. Challenges in evaluating surgical innovation. Lancet. 2009; 374:1097–1104. PMID:
19782875.

15. Garas G, Cingolani I, Panzarasa P, Darzi A, Athanasiou T. Network analysis of surgical innovation: measuring value and the virality of diffusion in robotic surgery. PLoS One. 2017; 12:e0183332. PMID:
28841648.

16. Rutten MJ, van Meurs HS, van de Vrie R, Gaarenstroom KN, Naaktgeboren CA, van Gorp T, et al. Laparoscopy to predict the result of primary cytoreductive surgery in patients with advanced ovarian cancer: a randomized controlled trial. J Clin Oncol. 2017; 35:613–621. PMID:
28029317.

17. Böhm S, Faruqi A, Said I, Lockley M, Brockbank E, Jeyarajah A, et al. Chemotherapy response score: development and validation of a system to quantify histopathologic response to neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J Clin Oncol. 2015; 33:2457–2463. PMID:
26124480.

18. Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations--part I. Gynecol Oncol. 2016; 140:313–322. PMID:
26603969.
19. Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations--part II. Gynecol Oncol. 2016; 140:323–332. PMID:
26757238.
20. Aletti GD, Santillan A, Eisenhauer EL, Hu J, Aletti G, Podratz KC, et al. A new frontier for quality of care in gynecologic oncology surgery: multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecol Oncol. 2007; 107:99–106. PMID:
17602726.

21. Lucidi A, Chiantera V, Gallotta V, Ercoli A, Scambia G, Fagotti A. Role of robotic surgery in ovarian malignancy. Best Pract Res Clin Obstet Gynaecol. 2017; 45:74–82. PMID:
28602522.

22. Magrina JF, Zanagnolo V, Noble BN, Kho RM, Magtibay P. Robotic approach for ovarian cancer: perioperative and survival results and comparison with laparoscopy and laparotomy. Gynecol Oncol. 2011; 121:100–105. PMID:
21194736.

23. Nezhat FR, Finger TN, Vetere P, Radjabi AR, Vega M, Averbuch L, et al. Comparison of perioperative outcomes and complication rates between conventional versus robotic-assisted laparoscopy in the evaluation and management of early, advanced, and recurrent stage ovarian, fallopian tube, and primary peritoneal cancer. Int J Gynecol Cancer. 2014; 24:600–607. PMID:
24557439.

24. Feuer GA, Lakhi N, Barker J, Salmieri S, Burrell M. Perioperative and clinical outcomes in the management of epithelial ovarian cancer using a robotic or abdominal approach. Gynecol Oncol. 2013; 131:520–524. PMID:
24080421.

25. Ackroyd SA, Thomas S, Angel C, Moore R, Meacham PJ, DuBeshter B. Interval robotic cytoreduction following neoadjuvant chemotherapy in advanced ovarian cancer. J Robot Surg. 2018; 12:245–250. PMID:
28631233.

26. Tozzi R, Gubbala K, Majd HS, Campanile RG. Interval laparoscopic en-bloc resection of the pelvis (L-EnBRP) in patients with stage IIIC-IV ovarian cancer: description of the technique and surgical outcomes. Gynecol Oncol. 2016; 142:477–483. PMID:
27450637.

27. Favero G, Macerox N, Pfiffer T, Köhler C, da Costa Miranda V, Estevez Diz MP, et al. Oncologic concerns regarding laparoscopic cytoreductive surgery in patients with advanced ovarian cancer submitted to neoadjuvant chemotherapy. Oncology. 2015; 89:159–166. PMID:
25968072.

28. Corrado G, Mancini E, Cutillo G, Baiocco E, Vici P, Sergi D, et al. Laparoscopic debulking surgery in the management of advanced ovarian cancer after neoadjuvant chemotherapy. Int J Gynecol Cancer. 2015; 25:1253–1257. PMID:
26111273.

29. Cardenas-Goicoechea J, Wang Y, McGorray S, Saleem MD, Carbajal Mamani SL, Pomputius AF, et al. Minimally invasive interval cytoreductive surgery in ovarian cancer: systematic review and meta-analysis. J Robot Surg. 2019; 13:23–33. PMID:
29992404.

30. Brown J, Drury L, Crane EK, Anderson WE, Tait DL, Higgins RV, et al. When less is more: minimally invasive surgery compared with laparotomy for interval debulking after neoadjuvant chemotherapy in women with advanced ovarian cancer. J Minim Invasive Gynecol. 2019; 26:902–909. PMID:
30240899.

31. Fagotti A, Gueli Alletti S, Corrado G, Cola E, Vizza E, Vieira M, et al. The INTERNATIONAL MISSION study: minimally invasive surgery in ovarian neoplasms after neoadjuvant chemotherapy. Int J Gynecol Cancer. 2019; 29:5–9. PMID:
30640676.