Abstract
A 50-year-old non-Hispanic white Caucasian female was diagnosed with breast cancer and was subsequently found to possess the tumorigenic ataxia telangiectasia mutated (ATM) and PALB2 variants but not the BRCA1 and BRCA2 variants. She visited the gynecologic oncology office for routine counseling about risk-reducing salpingo-oophorectomy (RRSO). Although the patient was asymptomatic, an adnexal mass was discovered in the physical examination performed by palpation. Upon using pre-operative imaging techniques, an 8 cm complex adnexal mass was identified. Her CA-125 level was elevated. She underwent complete cytoreductive surgery. Pathological analysis showed a stage IC clear cell carcinoma of the left ovary; subsequently, she received 6 cycles of adjuvant chemotherapy with a combination of carboplatin and paclitaxel. The patient exhibited no signs ovarian cancer in a follow-up appointment after 32 months of treatment. However, bilateral RRSO is not recommended for patients positive for ATM and PALB2. Breast cancer patients with PALB2 and ATM mutations should extensively discuss the risks and benefits of RRSO in light of current data.
Go to : 
Young women with cancer, women with a family history of breast cancer, and all women with ovarian cancer should have genetic counseling and testing. The goal is to improve care, and personalized clinical management and treatment. There is insufficient evidence to recommend surgery to prevent ovarian cancer in patients with ataxia telangiectasia mutated (ATM) and PALB2 variants. We present a patient with both breast and ovarian cancer with abnormal genetic testing. We analyzed the literature with potential treatment options.
Go to : 
A 50-year-old non-Hispanic white Caucasian female went for a consultation after treatment of a ductal carcinoma in situ of the breast that was positive for the estrogen and progesterone receptors. A clinical geneticist analyzed her pedigree to discover that the patient is a single child. Her daughter and son are in their twenties and are healthy. Her mother and paternal grandmother were diagnosed with breast cancer at the age of 57 and 50, respectively, and her maternal grandmother suffered from colon cancer at the age of 55. Using next generation sequencing, her genome indicated the presence of a pathogenic deletion between exons 62–63 in the ATM gene and a variant of unknown significance (c.2840T>C [p.Leu947Ser]) in the PALB2 gene. The sequencing analysis was negative for BRCA1, BRCA2, and other known pathogenic variants for familial cancers.
Patient surgical history included a hysterectomy when she was 46 to remove benign fibroids, and a bilateral mastectomy, left sentinel node biopsy, and bilateral breast reconstruction using contralateral free deep inferior epigastric artery perforator flaps at the age of 49.
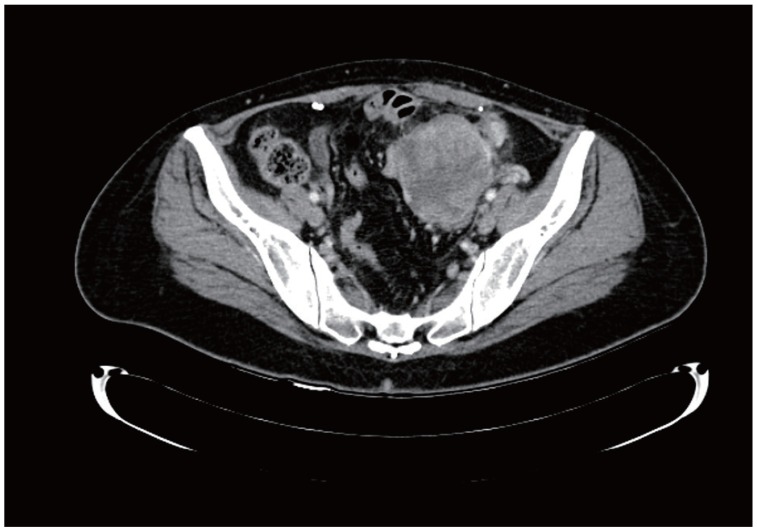
At the time of the consultation, the patient was clinically asymptomatic. A pelvic examination including palpation revealed an adnexal mass. Diagnostic workup including computed tomography showed a complex adnexal mass that was 8 cm long (Fig. 1) and elevated circulating levels of CA-125 (134 U/mL with a reference range of 0–35 U/mL). Differential diagnosis included endometrioma and an ovarian malignancy. Subsequently, the patient underwent complete cytoreductive surgery along with bilateral salpingo-oophorectomy, lymph node dissection, and omentectomy. The pathological results showed a stage IC clear cell carcinoma of the left ovary. Endometriosis was identified in the fallopian tubes and right ovary. Following this diagnosis, the patient received 6 cycles of adjuvant chemotherapy using a combination of carboplatin and paclitaxel. During a follow-up appointment after 32 months of treatment, the patient exhibited no symptoms of disease.
Go to : 
Ovarian cancer is the most lethal type of gynecologic cancer. In 2019, the American Cancer Society estimated 22,530 new ovarian cancer cases and 13,980 deaths due to ovarian cancer in the United States [1]. Women with breast cancer possessing the BRCA1 or BRCA2 pathogenic variants should consider risk-reducing salpingo-oophorectomy (RRSO) after childbirth or between the ages of 35–40 years for BRCA1 pathogenic variants, and 40–45 years for BRCA2 pathogenic variants [2]. The clinical management of non-BRCA genetic variants associated with increased risk for breast cancer remains unclear. However, breast cancer patients with a family history of breast cancer and PALB2/ATM mutations should extensively compare the risks and benefits of RRSO in light of current data. Germline mutations are risk factors for ovarian cancer whose clinical significance varies on race/ethnicity, family history, gravida and the existence of endometriosis, as it was in this case. Our case was associated with endometriosis. A meta-analysis suggests that the incidence of clear cell carcinoma is strongly associated with ovarian cancer (relative risk [RR], 2.2606; 95% confidence interval [CI], 2.225–3.053) and is one of the most common histological types [3]. Pregnancy reduces the risk of ovarian cancer risk by 45% (hazard ratio [HR], 0.55; 95% CI, 0.31–0.97). Each pregnancy is associated with an 11% reduction in the risk of developing ovarian cancer (HR, 0.89; 95% CI, 0.81–0.097) [4]. Race or ethnicity appears to play a role in the development of ovarian cancer. A study comprising 6,001 ovarian cancer patients by Kurian et al. [5] reported the presence of PALB2 variants of unknown significance in 7 non-Hispanic white (1.1%; 95% CI, 0.42–2.2), 1 black (1.7%; 95% CI, 0.04–9.2), 3 Asian (2.3%; 95% CI: 0.47–6.5), and 6 Hispanic patients (4.4%, 95% CI, 1.7–9.4). The PALB2 pathogenic variant was detected in 4 non-Hispanic white patients (0.6%; 95% CI, 0.16–1.5) and none in the other races.
Young women with cancer, women with a family history of breast cancer, and all women with ovarian cancer should be subjected to genetic counseling and testing [2]. Next generation sequencing of genomes is used by oncologists to determine the genetic basis for the disease that was suspected clinically or after pedigree analysis. The aim is to improve care through an accurate diagnosis, thereby allowing for more personalized clinical management and treatment.
Patients with proper knowledge provide consent to undergo genomic testing. The counseling session informing the patients of their genome results should be clear and clinically useful and misinterpretations caused by laboratory errors should be minimized. Three observations suggested the presence of a heritable cancer in our patient. First, the patient was relatively young and had a history of ductal cell carcinoma of the breast and clear cell carcinoma of the ovary. Second, the patient had a strong family history for cancer. Third, genomic analysis estimated a probability greater than 99% for carrying a pathogenic variant of the ATM gene with a variants of unknown significance in the PALB2 gene that did not allow for a confident conclusion of not having any health issues. In spite of these, we believe that further research and clinical reports are required before the ATM and PALB2 variants can be considered as risk factors for breast or ovarian cancer. The current National Comprehensive Cancer Network (NCCN) guidelines do not recommend RRSO in patients with any type of PALB2 or ATM variants [2]. Ramus et al. [6] have suggested that 9,000 ovarian cancer patients and 9,000 control individuals are needed to determine a significant association between PALB2 variants and ovarian cancer risk with 80% confidence.
PALB2 (partner and localizer of BRCA2) is a gene associated with Fanconi anemia; this protein binds to BRCA1 and BRCA2 at sites of DNA damage, thereby increasing the risk for breast cancer [7,8]. ATM is located on chromosome 11q22-23 and is fundamental in cellular DNA damage response. A deficiency in ATM is associated with breast cancer. Only a few studies have reported the frequency of PALB2 or ATM variants in patients with breast and ovarian cancer. The frequency of PALB2 variants in patients with ovarian cancer ranges between 0.21% and 1% [6,7,9-14]. Harter et al. [13] studied 523 ovarian cancer patients and found that only 5 patients (1%) had PALB2 variants, 1 patient (0.2%) possessed only the ATM variant, and 1 patient (0.2%) had both the PALB2 and ATM variants.
Antoniou et al. [8] estimated the RR of ovarian cancer among PALB2 mutation carriers to be 2.31 (95% CI, 0.77–6.97; P=0.18). Norquist et al. [7] reported a significantly increased risk of ovarian cancer in patients with PALB2 variants among a cohort of 1,915 ovarian cancer patients with two control groups (Exome Sequencing Project [ESP] dataset, odds ratio [OR], 10.2; 95% CI, 2.2–47; P<0.001 and Exome Aggregation group data set, OR, 4.4; 95% CI, 2.1–9.1; P<0.01). Kurian et al. [15] performed a control case-study that included 95,561 women (715 mutations in 701 [14%] ovarian cancer-inflicted patients) and did not find a link between ovarian cancer and the PALB2 variants (OR, 1.60; 95% CI, 0.98–2.60; P=0.058); however, they found a significant association with ATM variants (OR, 1.69; 95% CI, 1.19–2.40; P=0.0032). Kotsopoulos et al. [14] reported a study comprising 1,421 ovarian cancer patients and 4,300 European controls from the National Heart, Lung, and Blood Institute's ESP dataset and found no significance between the increase in ovarian cancer and PALB2 variants (OR, 4.55; 95% CI, 0.76–27.24; P=0.10). Ramus et al. [6] performed a study that included 3,374 patients with ovarian cancer and 3,487 control patients compiled from eight ovarian cancer-control studies; no link was found between the incidence of ovarian cancer and PALB2 (9 ovarian cancer cases compared to 3 in control, P=0.08). However, Lu et al. [9] performed whole-exome sequencing analysis for 11,416 patients with clinical symptoms of breast cancer, ovarian cancer, or both and compared the results with those from 3,988 control individuals. They found a significant association between the occurrence of breast cancer and PALB2 variants (OR, 5.53; 95% CI, 2.24–17.65) and ATM variants (OR, 2.97; 95% CI, 1.67–5.68). Furthermore, both breast and ovarian cancers were associated with the presence of ATM variants (OR, 2.85; 95% CI, 1.30–6.32) [9].
Finally, high-grade serous is the most common type of epithelial ovarian cancer is (70–80%) and clear cell carcinoma is least frequent (10%). Carter et al. [10] studied 2,801 patients with ovarian cancer to find that a pathogenic variant was identified in 14.7% and 8.9% cases of serous and clear cell carcinoma, respectively. Norquist et al. [7] reported that ovarian cancer patients with clear cell carcinoma had a lower overall frequency for mutations compared to high-grade serous (8.6% vs. 19.6%, P=0.04). Among 12 patients with PALB2 variants, 9 were found to have serous carcinoma and 1 patient had the clear cell type.
In conclusion, we hereby present a 50-year-old female with family and personal history of breast cancer with the absence of BRCA1 and BRCA2 pathogenic variants, but possessing a pathogenic deletion in the ATM gene and a variant of unknown significance in the PALB2 gene that was associated with the incidental finding of ovarian cancer. Combining our case report with a literature review suggests a link between the ATM/PALB2 variants and heritable breast and/or ovarian cancers. Further research and clinical reports are necessary before assigning ATM and PALB2 variants as risk factors for breast or ovarian cancer. However, it is reasonable to discuss the potential options with patients to prevent ovarian cancer. Based on published data and appropriate counseling, we recommend patients between 40–45 years of age to undergo RRSO. In accordance with Carter et al, the NCCN guidelines for ovarian cancer risk reduction associated with the ATM and PALB2 mutations may change in the future with more data [10].
Go to : 
Notes
Ethical approval: The study did not required approved by the Institutional Review Board of University of Florida, but required patient consent.
Patient consent: The patients provided written informed consent for the publication and the use of their images.
Go to : 
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34. PMID: 30620402.

2. NCCN. Genetic/familial high-risk assessment: breast and ovarian cancer. Version 2.2019. Fort Washington (PA): National Comprehensive Cancer Network;2018. 7. 30.
3. Kim HS, Kim TH, Chung HH, Song YS. Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis. Br J Cancer. 2014; 110:1878–1890. PMID: 24518590.

4. Gay GM, Lim JS, Chay WY, Chow KY, Tan MH, Lim WY. Reproductive factors, adiposity, breastfeeding and their associations with ovarian cancer in an Asian cohort. Cancer Causes Control. 2015; 26:1561–1573. PMID: 26342607.

5. Kurian AW, Ward KC, Howlader N, Deapen D, Hamilton AS, Mariotto A, et al. Genetic testing and results in a population-based cohort of breast cancer patients and ovarian cancer patients. J Clin Oncol. 2019; 37:1305–1315. PMID: 30964716.

6. Ramus SJ, Song H, Dicks E, Tyrer JP, Rosenthal AN, Intermaggio MP, et al. Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. J Natl Cancer Inst. 2015; 107:djv214. PMID: 26315354.
7. Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016; 2:482–490. PMID: 26720728.

8. Antoniou AC, Casadei S, Heikkinen T, Barrowdale D, Pylkäs K, Roberts J, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med. 2014; 371:497–506. PMID: 25099575.

9. Lu HM, Li S, Black MH, Lee S, Hoiness R, Wu S, et al. Association of breast and ovarian cancers with predisposition genes identified by large-scale sequencing. JAMA Oncol. 2019; 5:51–57. PMID: 30128536.

10. Carter NJ, Marshall ML, Susswein LR, Zorn KK, Hiraki S, Arvai KJ, et al. Germline pathogenic variants identified in women with ovarian tumors. Gynecol Oncol. 2018; 151:481–488. PMID: 30322717.

11. Lilyquist J, LaDuca H, Polley E, Davis BT, Shimelis H, Hu C, et al. Frequency of mutations in a large series of clinically ascertained ovarian cancer cases tested on multi-gene panels compared to reference controls. Gynecol Oncol. 2017; 147:375–380. PMID: 28888541.

12. Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. 2011; 108:18032–18037. PMID: 22006311.

13. Harter P, Hauke J, Heitz F, Reuss A, Kommoss S, Marmé F, et al. Prevalence of deleterious germline variants in risk genes including BRCA1/2 in consecutive ovarian cancer patients (AGO-TR-1). PLoS One. 2017; 12:e0186043. PMID: 29053726.
14. Kotsopoulos J, Sopik V, Rosen B, Fan I, McLaughlin JR, Risch H, et al. Frequency of germline PALB2 mutations among women with epithelial ovarian cancer. Fam Cancer. 2017; 16:29–34. PMID: 27631815.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download