1. Cunningham FG. Williams obstetrics. New York (NY): McGraw-Hill Education;2018.
3. Melchior H, Kurch-Bek D, Mund M. The prevalence of gestational diabetes: a population-based analysis of a nationwide screening program. Dtsch Arztebl Int. 2017; 114:412–418. PMID:
28669379.
4. Rahimi M, Karami Moghadam F. The prevalence of gestational diabetes mellitus and its related risk factors using one-step method in Kermanshah, 2016. Iran J Obstet Gynecol Infertil. 2017; 20:1–4.
5. Ramezani S, Ahmadi M, Saqhafi H, Alipoor M. Association of pregnancy-associated plasma protein a (PAPP-A) and gestational diabetes. Iran J Obstet Gynecol Infertil. 2017; 20:61–69.
6. Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018; 131:e49–64. PMID:
29370047.
7. Kebapcilar L, Kebapcilar AG, Ilhan TT, Ipekci SH, Baldane S, Pekin A, et al. Is the mean platelet volume a predictive marker of a low apgar score and insulin resistance in gestational diabetes mellitus? A retrospective case-control study. J Clin Diagn Res. 2016; 10:OC06–10.
8. Gorgal R, Gonçalves E, Barros M, Namora G, Magalhães A, Rodrigues T, et al. Gestational diabetes mellitus: a risk factor for non-elective cesarean section. J Obstet Gynaecol Res. 2012; 38:154–159. PMID:
21995455.

9. International Diabetes Federation. Madras Diabetes Research Foundation. Management of gestational diabetes in the community. Training manual for community health workers [Internet]. Brussels: International Diabetes Federation;Accessed 2018 Aug 4. Available from:
https://www.idf.org/e-library/guidelines.html.
10. Beneventi F, Simonetta M, Lovati E, Albonico G, Tinelli C, Locatelli E, et al. First trimester pregnancy-associated plasma protein-A in pregnancies complicated by subsequent gestational diabetes. Prenat Diagn. 2011; 31:523–528. PMID:
21404306.

11. Mertoglu C, Gunay M. Neutrophil-Lymphocyte ratio and Platelet-Lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab Syndr. 2017; 11(Suppl 1):S127–S131. PMID:
28017281.

13. Choi JL, Li S, Han JY. Platelet function tests: a review of progresses in clinical application. Biomed Res Int. 2014; 2014:456569. PMID:
24895576.

14. Sargın MA, Yassa M, Taymur BD, Celik A, Ergun E, Tug N. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios: are they useful for predicting gestational diabetes mellitus during pregnancy? Ther Clin Risk Manag. 2016; 12:657–665. PMID:
27217758.
15. Budak YU, Polat M, Huysal K. The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: a systematic review. Biochem Med (Zagreb). 2016; 26:178–193. PMID:
27346963.

16. McPherson RA, Pincus MR. Henry's clinical diagnosis and management by laboratory methods. St. Louis (MO): Elsevier;2017.
17. Zhou Z, Chen H, Sun M, Ju H.Mean platelet volume and gestational diabetes mellitus: a systematic review and meta-analysis. J Diabetes Res. 2018; 2018:1985026. PMID:
29854818.

18. Yang H, Zhu C, Ma Q, Long Y, Cheng Z.Variations of blood cells in prediction of gestational diabetes mellitus. J Perinat Med. 2015; 43:89–93. PMID:
24897392.

19. Chen X, Fang L, Lin H, Shen P, Zhang T, Li H, et al. The relationship between type 2 diabetes and platelet indicators. Iran J Public Health. 2017; 46:1211–1216. PMID:
29026786.
20. Jiang H, Yan WH, Li CJ, Wang AP, Dou JT, Mu YM. Elevated white blood cell count is associated with higher risk of glucose metabolism disorders in middle-aged and elderly Chinese people. Int J Environ Res Public Health. 2014; 11:5497–5509. PMID:
24852600.

21. Gorar S, Abanonu GB, Uysal A, Erol O, Unal A, Uyar S, et al. Comparison of thyroid function tests and blood count in pregnant women with versus without gestational diabetes mellitus. J Obstet Gynaecol Res. 2017; 43:848–854. PMID:
28194837.

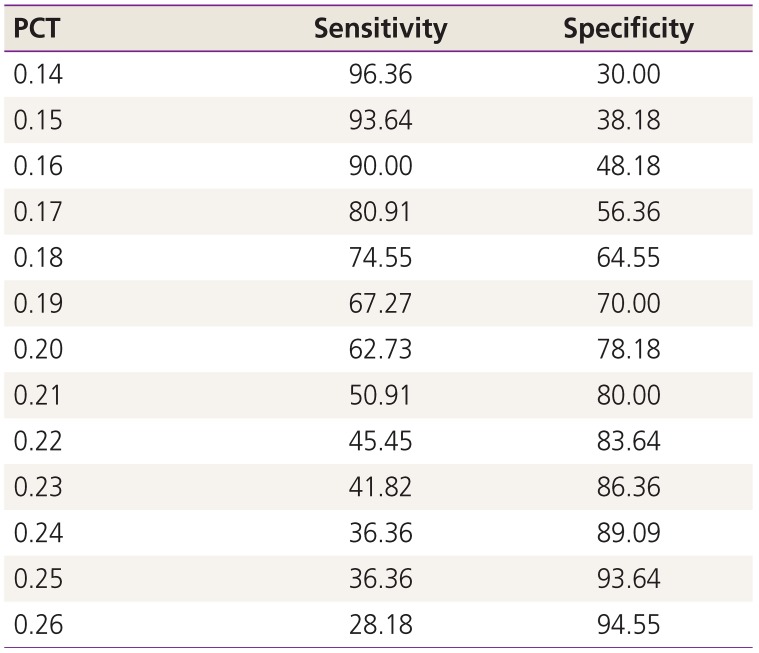
22. Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007; 96:644–647. PMID:
17376185.

23. Yun SH, Sim EH, Goh RY, Park JI, Han JY. Platelet activation: the mechanisms and potential biomarkers. BioMed Res Int. 2016; 2016:9060143. PMID:
27403440.

24. Sahbaz A, Cicekler H, Aynioglu O, Isik H, Ozmen U. Comparison of the predictive value of plateletcrit with various other blood parameters in gestational diabetes development. J Obstet Gynaecol. 2016; 36:589–593. PMID:
26758049.

25. Çeltik A, Akıncı B, Demir T. Mean platelet volume in women with gestational diabetes. Turk J Endocrinol Metab. 2016; 20:48–53.

26. Erdoğan S, Ozdemir O, Doğan HO, Sezer S, Atalay CR, Meriç F, et al. Liver enzymes, mean platelet volume, and red cell distribution width in gestational diabetes. Turk J Med Sci. 2014; 44:121–125. PMID:
25558571.

27. Chandrashekar V. Plateletcrit as a screening tool for detection of platelet quantitative disorders. J Hematol. 2013; 2:22–26.

28. Duman TT, Aktas G, Atak B, Kocak MZ. Is mean platelet volume to platelet ratio a promising indicator of diabetic regulation in type 2 diabetes mellitus? J Med Res. 2018; 4:137–139.

29. Shin DH, Rhee SY, Jeon HJ, Park JY, Kang SW, Oh J. An increase in mean platelet svolume/platelet count ratio is associated with vascular access failure in hemodialysis patients. PLoS One. 2017; 12:e0170357. PMID:
28095482.
30. Pattanathaiyanon P, Phaloprakarn C, Tangjitgamol S. Comparison of gestational diabetes mellitus rates in women with increased and normal white blood cell counts in early pregnancy. J Obstet Gynaecol Res. 2014; 40:976–982. PMID:
24612458.

31. Gomes CP, Torloni MR, Gueuvoghlanian-Silva BY, Alexandre SM, Mattar R, Daher S. Cytokine levels in gestational diabetes mellitus: a systematic review of the literature. Am J Reprod Immunol. 2013; 69:545–557. PMID:
23414425.

32. Catalano PM. Trying to understand gestational diabetes. Diabet Med. 2014; 31:273–281. PMID:
24341419.

33. Demir M, Duyuler PT, Guray U, Celik MC. Platelet to lymphocyte ratio on admission and prognosis in patients with acute cardiogenic pulmonary edema. J Emerg Med. 2018; 55:465–471. PMID:
30115388.

34. Agameya AM, Labib K, Moiety F. Using platelet-to-lymphocyte ratio as a diagnostic marker in malignant ovarian tumors. Int J Reprod Contracept Obstet Gynecol. 2018; 7:2089–2092.

35. Durmus E, Kivrak T, Gerin F, Sunbul M, Sari I, Erdogan O. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are predictors of heart failure. Arq Bras Cardiol. 2015; 105:606–613. PMID:
26536980.

36. Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017; 10:12. PMID:
28057051.

37. Saadati N, Anafcheh M, Ahmadzadeh B, Albookordi M, Najafian M. Effect of blood group, height, and weight gain during pregnancy on gestational diabetes mellitus. Iran J Obstet Gynecol Infertil. 2018; 21:34–42.
38. Shimodaira M, Yamasaki T, Nakayama T. The association of maternal ABO blood group with gestational diabetes mellitus in Japanese pregnant women. Diabetes Metab Syndr. 2016; 10:S102–5. PMID:
27025793.

39. Huidobro M A, Torres C D, Paredes F. Association of abo blood groups with gestational diabetes mellitus. Rev Med Chil. 2017; 145:431–435. PMID:
28748989.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



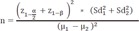
 XML Download
XML Download