Abstract
With technical advances in computed tomography and the introduction of non-ionic low- or iso-osmolar iodinated contrast media (ICM), the use of ICM and the occurrence of ICM-related hypersensitivity reactions (HSRs) has rapidly increased. Although ICM-related HSRs are known to be mild, they still represent life-threatening events in rare instances. It is therefore important to prevent recurrent HSRs in high-risk patients. Changing the culprit contrast agent is a powerful known tool for reducing the recurrence rate of HSRs. Based on the large body of evidence, the American College of Radiology manual on contrast media (latest version 10.3) suggests that changing the ICM within the same class may help reduce the likelihood of a subsequent contrast reaction. Furthermore, the European Society of Urogenital Radiology guidelines on contrast agents (latest version 10) also recommends using a different contrast agent with previous contrast agent reactors to reduce the risk of an acute reaction. In this article, we review the necessity and clinical efficacy of changing the culprit ICM for high-risk patients at the time of re-exposure to prevent ICM-related HSRs and minimize the risk of fatality.
Go to : 
REFERENCES
1. Ministry of Food and Drug Safety. 2014 Trend analysis of reporting drug safety information. Cheongju: Ministry of Food and Drug Safety;2015.
2. Shin MJ, Cho YJ. Management of adverse reaction to iodinated radiocontrast media. J Korean Med Assoc. 2012; 55:779–790.

3. Lasser EC, Lyon SG, Berry CC. Reports on contrast media reactions: analysis of data from reports to the U.S. Food and Drug Administration. Radiology. 1997; 203:605–610.

4. Wysowski DK, Nourjah P. Deaths attributed to X-ray contrast media on U.S. death certificates. AJR Am J Roentgenol. 2006; 186:613–615.

5. Wolf GL, Arenson RL, Cross AP. A prospective trial of ionic vs nonionic contrast agents in routine clinical practice: comparison of adverse effects. AJR Am J Roentgenol. 1989; 152:939–944.

6. Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990; 175:621–628.

7. Cha MJ, Kang DY, Lee W, Yoon SH, Choi YH, Byun JS, Lee J, Kim YH, Choo KS, Cho BS, Jeon KN, Jung JW, Kang HR. Hypersensitivity reactions to iodinated contrast media: a multicenter study of 196 081 patients. Radiology. 2019; 293:117–124.
8. American College of Radiology. ACR manual on contrast media. Version 10.3. Reston: American College of Radiology;2018.
9. Kobayashi D, Takahashi O, Ueda T, Deshpande GA, Arioka H, Fukui T. Risk factors for adverse reactions from contrast agents for computed tomography. BMC Med Inform Decis Mak. 2013; 13:18.

10. Abe S, Fukuda H, Tobe K, Ibukuro K. Protective effect against repeat adverse reactions to iodinated contrast medium: Premedication vs. changing the contrast medium. Eur Radiol. 2016; 26:2148–2154.

11. Park HJ, Park JW, Yang MS, Kim MY, Kim SH, Jang GC, Nam YH, Kim GW, Kim S, Park HK, Jung JW, Park JS, Kang HR. Re-exposure to low osmolar iodinated contrast media in patients with prior moderate-to-severe hypersensitivity reactions: a multicentre retrospective cohort study. Eur Radiol. 2017; 27:2886–2893.

12. Lee SY, Yang MS, Choi YH, Park CM, Park HW, Cho SH, Kang HR. Stratified premedication strategy for the prevention of contrast media hypersensitivity in high-risk patients. Ann Allergy Asthma Immunol. 2017; 118:339–344. .e1.

13. Park SJ, Kang DY, Sohn KH, Yoon SH, Lee W, Choi YH, Cho SH, Kang HR. Immediate mild reactions to CT with iodinated contrast media: strategy of contrast media readministration without corticosteroids. Radiology. 2018; 288:710–716.
14. European Society of Urogenital Radiology. ESUR guidelines on contrast agents. Version 10 [Internet]. Wien: European Society of Urogenital Radiology;2019. [cited 2020 Feb 14]. Available from:. http://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf.
15. Korean Society of Radiology; Korean Academy of Asthma, Allergy and Clinical Immunology. Korean clinical practice guideline on adverse reactions of injectable iodinated contrast agent and gadolinium contrast agent for MRI. 2nd edition.Seoul: Korean Society of Radiology;2016.
Go to : 
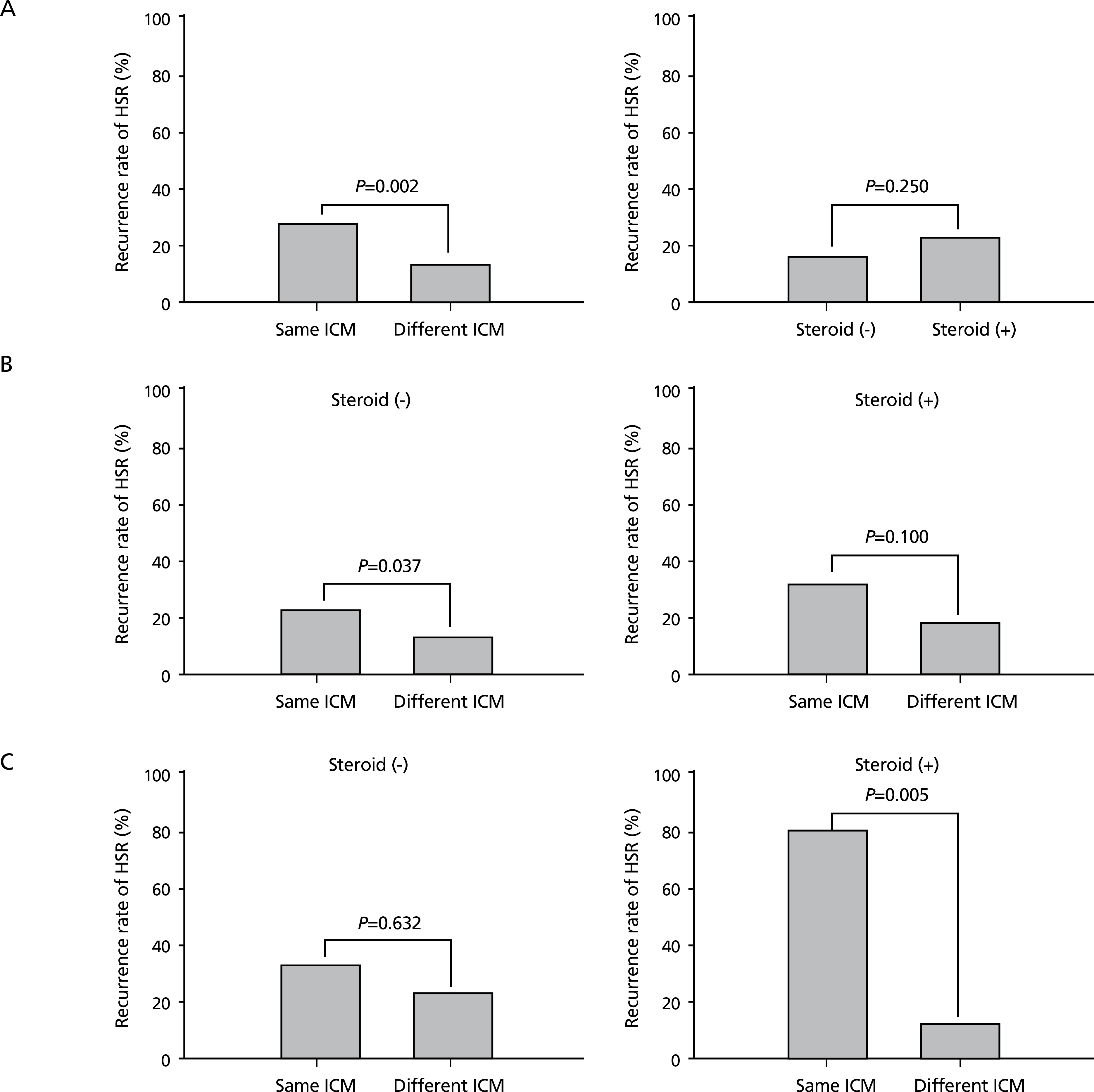 | Figure 1.Recurrence rates of hypersensitivity reactions (HSRs) according to iodinated contrast medium (ICM) changes and steroid premedication in the total patients (A), in patients with a moderate initial HSR (B). and in patients with a severe initial HSR (C). Reproduced from Park HJ et al. Eur Radiol 2017;27:2886-2893, with permission from Springer Nature [11]. |
Table 1.
Recurrence rates of iodinated contrast media-related hypersensitivity reaction according to the combination of contrast media in absence of premedication
| Combination of contrast media | Recurrence | Odds ratio a) | 95% confidence interval | P-value | Odds ratio b) | 95% confidence interval | P-value |
|---|---|---|---|---|---|---|---|
| Iopamidol/iopromide | 24/110 (21.8) | 0.470 | 0.247–0.892 | 0.021 | 1.863 | 0.995–3.487 | 0.052 |
| Iopamidol/iopromide | 11/58 (19.0) | 0.366 | 0.163–0.820 | 0.015 | 1.466 | 0.654–3.289 | 0.353 |
| Iohexol/iopromide | 37/268 (13.8) | 0.402 | 0.227–0.712 | 0.002 | 1.353 | 0.788–2.321 | 0.273 |
| Iobitridol/iohexol | 32/261 (12.3) | 0.245 | 0.127–0.474 | <0.001 | 0.877 | 0.468–1.680 | 0.713 |
| Iobitridol/iopromide | 18/169 (10.7) | 0.296 | 0.129–0.682 | 0.001 | 1.048 | 0.462–2.374 | 0.911 |
| Iobitridol/iopamidol | 7/17 (41.2) | 0.942 | 0.340–2.608 | 0.909 | 4.175 | 1.545–11.281 | 0.005 |
| Iobitridol/ioversol | 7/21 (33.3) | 0.890 | 0.321–2.466 | 0.823 | 3.310 | 1.210–9.056 | 0.020 |
| Iopromide/ioversol | 1/2 (50.0) | 1.344 | 0.083–21.898 | 0.835 | 5.961 | 0.369–96.259 | 0.208 |
| Iohexol/ioversol | 5/12 (41.7) | 0.876 | 0.237–3.232 | 0.842 | 3.585 | 0.978–13.137 | 0.054 |
| Iohexol/iomeprol | 3/13 (23.1) | 0.709 | 0.163–3.084 | 0.647 | 1.937 | 0.437–8.591 | 0.384 |
| Iomeprol/iopamidol | 4/19 (21.1) | 0.472 | 0.144–1.551 | 0.216 | 1.804 | 0.551–5.913 | 0.330 |
| Iopamidol/ioversol | 2/10 (20.0) | 0.188 | 0.023–1.569 | 0.123 | 0.779 | 0.094–6.454 | 0.817 |
| Iomeprol/iopromide | 2/11 (18.2) | 0.612 | 0.108–3.451 | 0.578 | 1.877 | 0.321–10.980 | 0.485 |
| Iobitridol/iomeprol | 0/8 (0) | NA | NA | NA | NA | NA | NA |
| Iomeprol/ioversol | 0/1 (0) | NA | NA | NA | NA | NA | NA |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download