1. Ou X, Hua Y, Liu J, Gong C, Zhao W. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ. 2017; 189(7):E260–E267. PMID:
28246239.

2. Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015; 372(23):2185–2196. PMID:
25981908.

3. Rochwerg B, Granton D, Wang DX, Helviz Y, Einav S, Frat JP, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019; 45(5):563–572. PMID:
30888444.

4. Yoo JW, Synn A, Huh JW, Hong SB, Koh Y, Lim CM. Clinical efficacy of high-flow nasal cannula compared to noninvasive ventilation in patients with post-extubation respiratory failure. Korean J Intern Med. 2016; 31(1):82–88. PMID:
26767861.

5. Kim HJ, Asai T. High-flow nasal oxygenation for anesthetic management. Korean J Anesthesiol. 2019; 72(6):527–547. PMID:
31163107.

6. Chung SM, Choi JW, Lee YS, Choi JH, Oh JY, Min KH, et al. Clinical effectiveness of high-flow nasal cannula in hypoxaemic patients during bronchoscopic procedures. Tuberc Respir Dis. 2019; 82(1):81–85.

7. Abdo WF, Heunks LM. Oxygen-induced hypercapnia in COPD: myths and facts. Crit Care. 2012; 16(5):323. PMID:
23106947.

8. Austin MA, Wills KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010; 341:c5462. PMID:
20959284.

9. Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017; 50(2):1602426. PMID:
28860265.

10. Pisani L, Vega ML. Use of nasal high flow in stable COPD: rationale and physiology. COPD. 2017; 14(3):346–350. PMID:
28459282.

11. Bräunlich J, Beyer D, Mai D, Hammerschmidt S, Seyfarth HJ, Wirtz H. Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration. 2013; 85(4):319–325. PMID:
23128844.

12. Rea H, McAuley S, Jayaram L, Garrett J, Hockey H, Storey L, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med. 2010; 104(4):525–533. PMID:
20144858.

13. Seo KW, Ahn JJ, Jegal Y, Ra SW, Bae S, Kim JH, et al. High-flow nasal cannula oxygen therapy for acute hypoxemic respiratory failure in patients with chronic lung disease in terms of hospital outcomes. Intensive Care Med. 2018; 44(3):387–388. PMID:
29322202.

14. Kim ES, Lee H, Kim SJ, Park J, Lee YJ, Park JS, et al. Effectiveness of high-flow nasal cannula oxygen therapy for acute respiratory failure with hypercapnia. J Thorac Dis. 2018; 10(2):882–888. PMID:
29607161.

15. Jeong JH, Kim DH, Kim SC, Kang C, Lee SH, Kang TS, et al. Changes in arterial blood gases after use of high-flow nasal cannula therapy in the ED. Am J Emerg Med. 2015; 33(10):1344–1349. PMID:
26319192.

16. Onodera Y, Akimoto R, Suzuki H, Nakane M, Kawamae K. A high-flow nasal cannula system set at relatively low flow effectively washes out CO
2 from the anatomical dead space of a respiratory-system model. Korean J Anesthesiol. 2017; 70(1):105–106. PMID:
28184277.
17. Demoule A, Girou E, Richard JC, Taillé S, Brochard L. Increased use of noninvasive ventilation in French intensive care units. Intensive Care Med. 2006; 32(11):1747–1755. PMID:
16799775.

18. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983; 70(1):41–55.

19. Lee HW, Choi SM, Lee J, Park YS, Lee CH, Yoo CG, et al. Reduction of PaCO
2 by high-flow nasal cannula in acute hypercapnic respiratory failure patients receiving conventional oxygen therapy. Acute Crit Care. 2019; 34(3):202–211. PMID:
31723929.
20. Lee MK, Choi J, Park B, Kim B, Lee SJ, Kim SH, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018; 12(6):2046–2056. PMID:
29392846.

21. Bräunlich J, Wirtz H. Nasal high-flow in acute hypercapnic exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018; 13:3895–3897. PMID:
30555226.

22. Jing G, Li J, Hao D, Wang T, Sun Y, Tian H, et al. Comparison of high flow nasal cannula with noninvasive ventilation in chronic obstructive pulmonary disease patients with hypercapnia in preventing postextubation respiratory failure: a pilot randomized controlled trial. Res Nurs Health. 2019; 42(3):217–225. PMID:
30887549.

23. Bello G, De Pascale G, Antonelli M. Noninvasive ventilation: practical advice. Curr Opin Crit Care. 2013; 19(1):1–8. PMID:
23235541.
24. Vadász I, Hubmayr RD, Nin N, Sporn PH, Sznajder JI. Hypercapnia: a nonpermissive environment for the lung. Am J Respir Cell Mol Biol. 2012; 46(4):417–421. PMID:
22246860.

25. Roca O, Hernández G, Díaz-Lobato S, Carratalá JM, Gutiérrez RM, Masclans JR. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care. 2016; 20(1):109. PMID:
27121707.

26. Azoulay E, Lemiale V, Mokart D, Nseir S, Argaud L, Pène F, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. 2018; 320(20):2099–2107. PMID:
30357270.
27. Fernandez R, Subira C, Frutos-Vivar F, Rialp G, Laborda C, Masclans JR, et al. High-flow nasal cannula to prevent postextubation respiratory failure in high-risk non-hypercapnic patients: a randomized multicenter trial. Ann Intensive Care. 2017; 7(1):47. PMID:
28466461.

28. Hernández G, Vaquero C, González P, Subira C, Frutos-Vivar F, Rialp G, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016; 315(13):1354–1361. PMID:
26975498.
29. Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016; 316(15):1565–1574. PMID:
27706464.
30. Millar J, Lutton S, O'Connor P. The use of high-flow nasal oxygen therapy in the management of hypercarbic respiratory failure. Ther Adv Respir Dis. 2014; 8(2):63–64. PMID:
24670392.

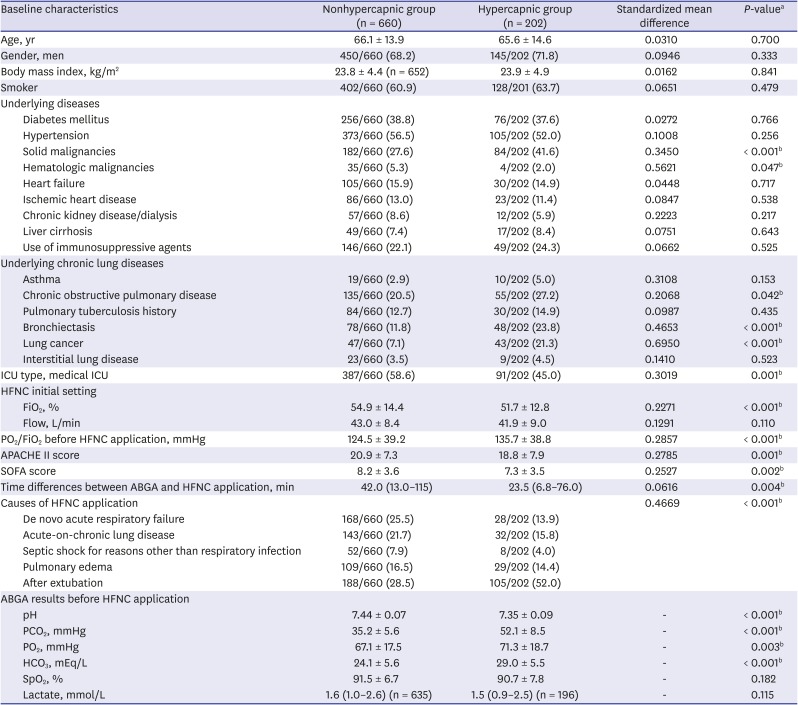
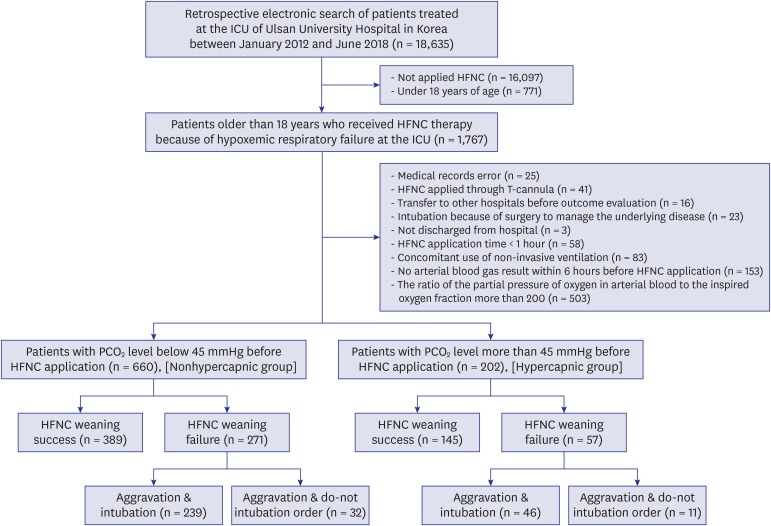
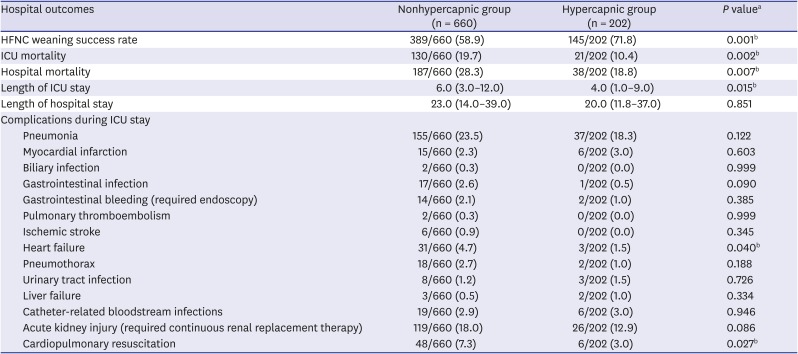
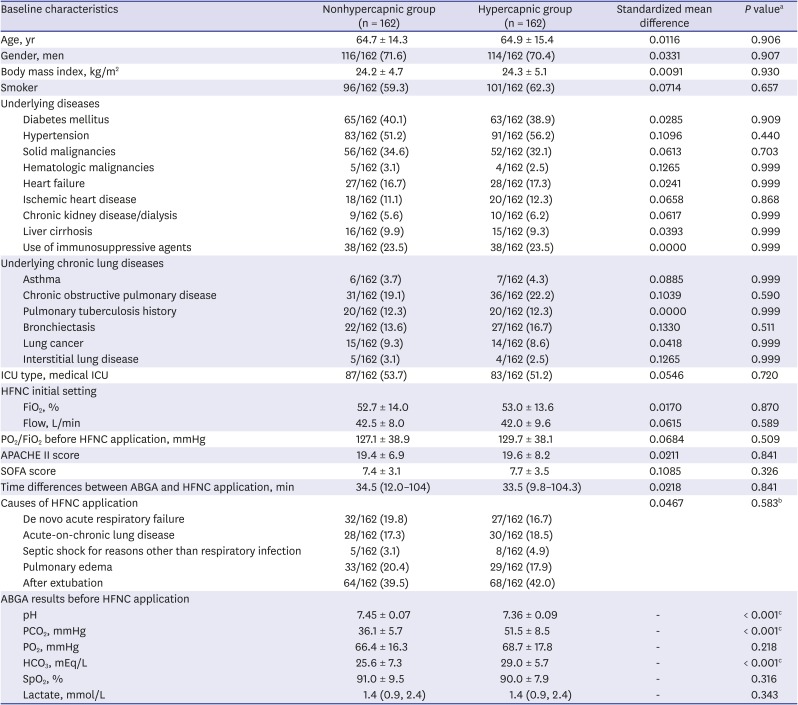




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download