This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Familial Mediterranean fever (FMF) is an autoinflammatory disease that has self-limiting inflammatory attacks during polyserositis. Hepcidin is a protein, and interleukin-6 stimulation increases hepcidin levels. Calprotectin (CLP) is a recently defined cytokine released from monocytes and neutrophils in response to tissue trauma and inflammation. There are studies in the literature showing that it can be used as a biomarker in rheumatic diseases such as ankylosing spondylitis and rheumatoid arthritis. Here, we compared the levels of hepcidin and CLP in healthy individuals and FMF patients during an attack-free period and show its relation to genetic mutations.
Methods
This is a cross-sectional study. Between July 2017 and December 2017, 60 patients diagnosed with FMF an admitted to the Cumhuriyet University Faculty of Medicine Department of Internal Medicine Rheumatology as well as 60 healthy volunteers without any rheumatic, systemic, or metabolic diseases were enrolled in this study. Blood was collected from a peripheral vein to measure serum CLP and hepcidin levels. Blood tests were examined by ELISA; the study protocol was approved by the local ethics committee.
Results
Median serum hepcidin level was 468.1 (210.3–807.8) pg/mL in FMF group and 890.0 (495.0–1,716.9) pg/mL in the healthy control (HC) group. There was a statistically significant difference between the two groups (P < 0.001). The median serum levels of CLP in the FMF group were measured as 1,331.4 (969.3–1,584.6 pg/mL and 73.8(45.0–147.9) pg/mL in the HC group. There was a statistically significant difference between the two groups (P < 0.001). Receiver operating characteristic analysis showed that the sensitivity was 66.7% and the specificity was 71.7% at serum hepcidin < 581.25 pg/mL (P < 0.05); the sensitivity was 96.7% and specificity was 100% at CLP > 238 pg/mL (P < 0.05). There was no significant difference between serum hepcidin and CLP levels in FMF patients with M694V homozygous and M694V heterozygous (P > 0.05). There was no significant difference in serum hepcidin levels between FMF patients with and without arthritis, proteinuria, and amyloidosis (P < 0.05). There was no significant correlation between laboratory findings, gender, age, and serum CLP and hepcidin levels (P > 0.05, r < 0.25).
Conclusion
Serum CLP levels in FMF patients during an attack-free period are significantly higher than in the HC groups. Serum hepcidin levels in FMF patients are significantly lower than in the HC group. Low levels of hepcidin may be explained by including FMF patients during an attack-free period in the study. CLP may be an important biomarker in FMF. A better understanding of the role of these biomarkers in the diagnosis of FMF is needed to evaluate the results in a more comprehensive way.
Keywords: FMF, Calprotectin, Hepcidin
INTRODUCTION
Familial Mediterranean fever (FMF) is an auto-inflammatory disease manifesting inflammatory attacks of peritonitis, pleuritis, and pericarditis accompanied by fever and arthritis.
1 There are two types of attacks: typical and atypical. Typical attacks are characterized by symptoms such as classic periods of fever and abdominal pain. Fever periods are less frequent in atypical attacks but there may be more arthritis and myalgia.
2 Although FMF has criteria for classification of Tel-Hashomer and Livneh, it is not so easy to diagnose in each patient. The most dangerous complication in untreated FMF patients is the development of amyloidosis and consequent renal failure. Subclinical inflammation during an attack-free period is a risk factor for amyloidosis.
Hepcidin is a peptide synthesized by the liver.
3 Insufficient hepcidin synthesis leads to iron accumulation in the body while excessive increase of hepcidin synthesis causes iron deficiency anemia. Interleukin-6 (IL-6) synthesized during inflammation increases hepcidin production whereas IL-6 antibody reduces hepcidin synthesis.
4 The role of hepcidin in infections is clear. There are also studies on the role of hepcidin in autoimmune diseases in the literature such as rheumatoid arthritis (RA), pro-hepcidin may be a marker indicating disease activity.
5 Another study suggested that hepcidin level may be associated with disease activity in patients with systemic lupus erythematosus (SLE).
6 However, there are no studies reporting hepcidin levels in FMF patients.
Calprotectin (CLP; S100A8/A9) is a newly identified cytokine that can be detected in the cytoplasm of healthy individuals. However, it increases in response to tissue trauma and inflammation. CLP is released from activated neutrophil and macrophages during inflammation creates a pro-inflammatory effect.
7 The release of CLP is increased as a result of the stimulation of damage-associated molecular patterns and pathogen-associated molecular patterns. CLP plays an active role in the innate immune system. However, it also plays a role between natural and adaptive immunity. CLP is particularly involved in the chemotaxis stage.
8 Many recent studies have shown that CLP is a potentially more sensitive biomarker of rheumatic diseases than inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).
CLP was found to be high in the serum and synovium of RA patients.
9 Previous studies in FMF patients found high fecal CLP.
10 However, there are no studies of both biomarkers evaluated together in FMF patients. Subclinical inflammation may be present in FMF patients. The diagnostic criteria are still being developed, but there are no specific markers for diagnosis. Here, we aimed to demonstrate the availability of CLP and hepcidin in FMF patients to demonstrate the presence of subclinical inflammation—especially during the attack-free period.
METHODS
This study was conducted in Cumhuriyet University Faculty of Medicine, Department of Internal Medicine, Division of Rheumatology.
We recruited patients who were admitted to Cumhuriyet University Faculty of Medicine Hospital Department of Internal Medicine Rheumatology outpatient clinic between August 2017 and February 2018 and who were diagnosed with FMF according to the Tel-Hashomer criteria. The healthy subjects included in the control group were selected among the individuals who applied to Cumhuriyet University Faculty of Medicine Hospital Department of Internal Medicine General Internal Medicine outpatient clinic. The inclusion criteria were 18–65 years of age, no known neuropsychiatric disease, no active infection during the study, and no additional rheumatic disease (e.g., RA, SLE). The exclusion criterion was having rheumatic disease other than FMF in the patient group. The exclusion criteria in the control group were a history of malignancy, active infection, and having rheumatic disease. The patients were selected from those who were in the attack-free period. Sixty patients with FMF and 60 healthy subjects who met the inclusion criteria were included.
The demographic characteristics of both groups as well as information on their diagnoses, disease courses, and treatments were obtained from the patient files and interviews. In addition, laboratory parameters of the patients and information on the treatments they received were recorded. The blood samples were collected from a peripheral vein and centrifuged at 5,000 G for 15 minutes and stored in an Eppendorf tube at −80°C. The samples were then studied by ELISA method in CÜTFH Division of Biochemistry. A Cloud Clone® (Katy, TX, USA) ELISA Kit and Cloud Clone® ELISA Kit were used for measuring hepcidin and CLP, respectively. In addition, routine examinations of the patients (MEFV gene, creatinine, alanine aminotransferase, aspartate transaminase, fibrinogen, CRP, hemogram parameters, ESR, ferritin, iron, and iron binding capacity) in the outpatient clinic settings were also recorded using a file scanning method.
Statistical analysis
Statistical assessments were performed using SPSS 22 (IBM Corp., Armonk, NY, USA) software. Whether the data showed normal distribution was evaluated using histogram, q-q plots, and a Shapiro-Wilk test. The mean values were used for the parameters with normal distribution, and median values were used for those without normal distribution. The homogeneity of variance was tested by the Levene's test. For two-group comparisons, a Mann-Whitney U test and two independent sample t-tests were used for quantitative variables. A Pearson χ2 analysis was used to compare the categorical data. The correlation between quantitative data was evaluated via a Spearman correlation analysis. Receiver operating characteristic (ROC) analysis investigated hepcidin and CLP variables on FMF status. The area under the ROC curve were calculated with a 95% confidence interval (CI). The cut-off values for each marker were calculated with Youden's index. The areas between the ROC curves were compared using the DeLong method. The sensitivity, specificity, positive predictive value, and negative predictive value for optimum cut-off values were in the 95% CI. The significance level was P < 0.05.
Ethics statement
Ethical committee approval was obtained from Cumhuriyet University Faculty of Medicine Clinical Trials Ethics Committee with the decision dated 17.01.2017 and numbered 2017-01/06. The study was conducted in accordance with the principles of the Helsinki Declaration of the World Medical Association. All participants gave informed written consent.
RESULTS
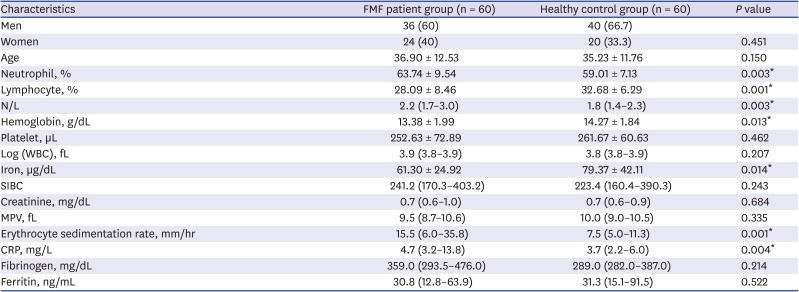
We enrolled 60 patients with FMF and 60 healthy controls (HCs). Age and gender characteristics were similar in both groups. There was no significant difference between the two groups in terms of serum creatinine, platelet, white blood cell (WBC) counts, leukocyte count WBC, fibrinogen, ferritin, and mean platelet volume. The values that are higher in the patient group were in the laboratory reference ranges; the neutrophil, lymphocyte, neutrophil lymphocyte ratio, hemoglobin, ESR, and CRP levels were significantly different between the two groups (
Table 1). All patients were using colchicine. Arthritis and other patient characteristics are summarized in
Table 2.
Table 1
Demographic data and laboratory values
|
Characteristics |
FMF patient group (n = 60) |
Healthy control group (n = 60) |
P value |
|
Men |
36 (60) |
40 (66.7) |
|
|
Women |
24 (40) |
20 (33.3) |
0.451 |
|
Age |
36.90 ± 12.53 |
35.23 ± 11.76 |
0.150 |
|
Neutrophil, % |
63.74 ± 9.54 |
59.01 ± 7.13 |
0.003*
|
|
Lymphocyte, % |
28.09 ± 8.46 |
32.68 ± 6.29 |
0.001*
|
|
N/L |
2.2 (1.7–3.0) |
1.8 (1.4–2.3) |
0.003*
|
|
Hemoglobin, g/dL |
13.38 ± 1.99 |
14.27 ± 1.84 |
0.013*
|
|
Platelet, µL |
252.63 ± 72.89 |
261.67 ± 60.63 |
0.462 |
|
Log (WBC), fL |
3.9 (3.8–3.9) |
3.8 (3.8–3.9) |
0.207 |
|
Iron, µg/dL |
61.30 ± 24.92 |
79.37 ± 42.11 |
0.014*
|
|
SIBC |
241.2 (170.3–403.2) |
223.4 (160.4–390.3) |
0.243 |
|
Creatinine, mg/dL |
0.7 (0.6–1.0) |
0.7 (0.6–0.9) |
0.684 |
|
MPV, fL |
9.5 (8.7–10.6) |
10.0 (9.0–10.5) |
0.335 |
|
Erythrocyte sedimentation rate, mm/hr |
15.5 (6.0–35.8) |
7.5 (5.0–11.3) |
0.001*
|
|
CRP, mg/L |
4.7 (3.2–13.8) |
3.7 (2.2–6.0) |
0.004*
|
|
Fibrinogen, mg/dL |
359.0 (293.5–476.0) |
289.0 (282.0–387.0) |
0.214 |
|
Ferritin, ng/mL |
30.8 (12.8–63.9) |
31.3 (15.1–91.5) |
0.522 |
Table 2
Some clinical and disease characteristics of patients
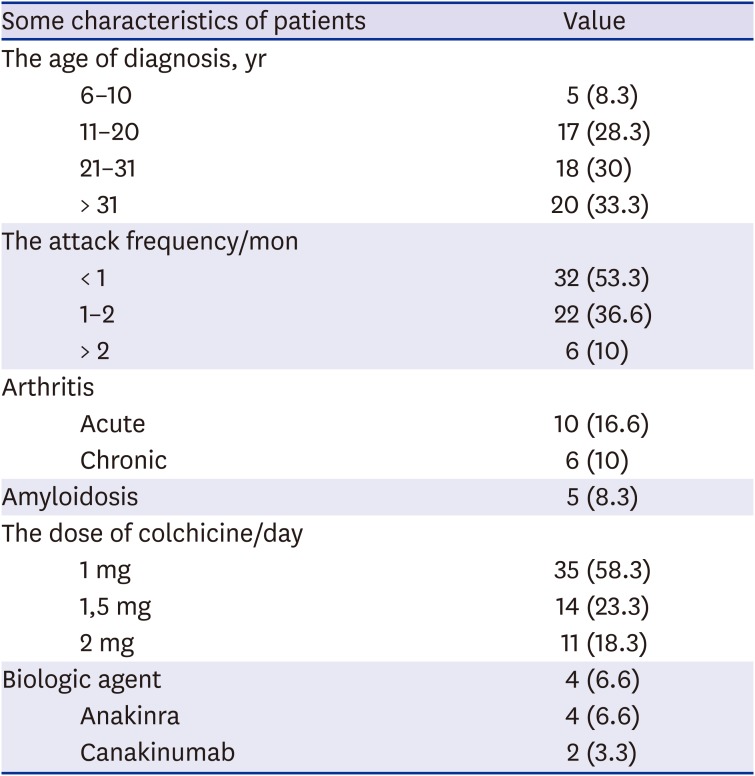
|
Some characteristics of patients |
Value |
|
The age of diagnosis, yr |
|
|
6–10 |
5 (8.3) |
|
11–20 |
17 (28.3) |
|
21–31 |
18 (30) |
|
> 31 |
20 (33.3) |
|
The attack frequency/mon |
|
|
< 1 |
32 (53.3) |
|
1–2 |
22 (36.6) |
|
> 2 |
6 (10) |
|
Arthritis |
|
|
Acute |
10 (16.6) |
|
Chronic |
6 (10) |
|
Amyloidosis |
5 (8.3) |
|
The dose of colchicine/day |
|
|
1 mg |
35 (58.3) |
|
1,5 mg |
14 (23.3) |
|
2 mg |
11 (18.3) |
|
Biologic agent |
4 (6.6) |
|
Anakinra |
4 (6.6) |
|
Canakinumab |
2 (3.3) |
The
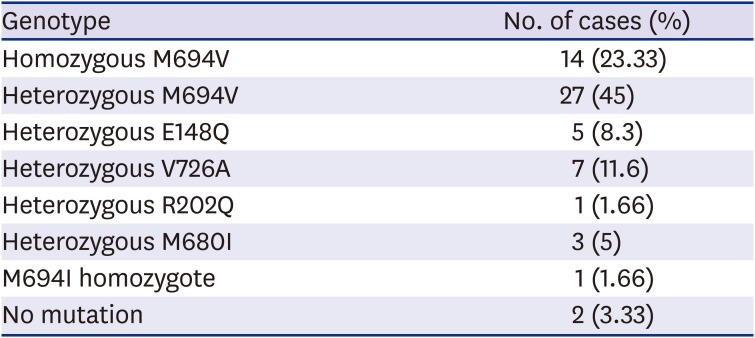
MEFV gene mutation analysis showed that a homozygous M694V mutation was detected in 14 (23.33%) people, heterozygous M694V in 27 (45%) people, heterozygous E148Q in five (8.3%) people, heterozygous V726A in 7 (11.66%) people, heterozygous R202Q in 1 (1.66%) people, heterozygous M680I in three (5%), and homozygous M694I in one (1.66%). No mutation was detected in the
MEFV gene in two (3.33%) patients (
Table 3).
Table 3
Distribution of MEFV genetic mutations in familial Mediterranean fever patient group
|
Genotype |
No. of cases (%) |
|
Homozygous M694V |
14 (23.33) |
|
Heterozygous M694V |
27 (45) |
|
Heterozygous E148Q |
5 (8.3) |
|
Heterozygous V726A |
7 (11.6) |
|
Heterozygous R202Q |
1 (1.66) |
|
Heterozygous M680I |
3 (5) |
|
M694I homozygote |
1 (1.66) |
|
No mutation |
2 (3.33) |
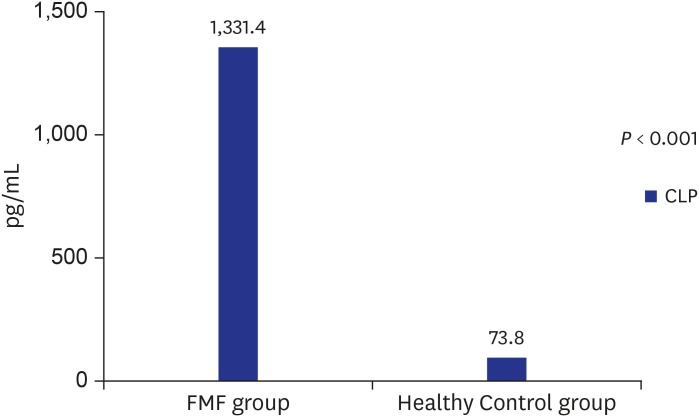
CLP and hepcidin values
The median serum hepcidin level was 468.1 (210.3–807.8) pg/mL in the FMF group and 890.0 (495.0–1,716.9) pg/mL in the control group. There was a statistically significant difference between the two groups (
P < 0.001) (
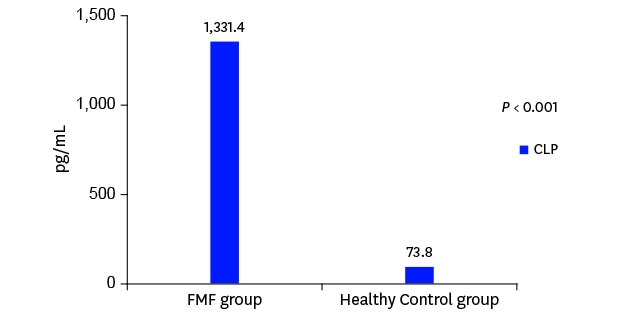
Fig. 1). The median serum CLP level was measured as 1,331.4 (969.3–1,584.6) pg/mL in the FMF group and 73.8 (45.0–147.9) pg/mL in the control group. There was a statistically significant difference between the two groups (
P < 0.001) (
Fig. 2).
Fig. 1
Serum hepcidin levels in FMF patients and control group.
FMF = familial Mediterranean fever.

Fig. 2
Serum CLP levels in FMF patients and control group.
FMF = familial Mediterranean fever, CLP = calprotectin.
ROC analysis results (
Supplementary Fig. 1) shows that the area under the curve for the hepcidin value was 0.721; this was statistically significant (
P < 0.001). The value of 54.03 pg/mL was determined to have 65% sensitivity and 68.3% specificity. The positive predictive value was 70.2%, and the negative predictive value was 68.3% (
Table 4).
Table 4
ROC analysis values

|
Marker |
ROC statistics |
Diagnostic statistics |
|
AUC |
P value |
Sensitivity (%) |
Specificity (%) |
Positive predictive value (%) |
Negative predictive value (%) |
|
Hepcidin < 581.25 pg/mL |
0.721 (0.629–0.813) |
< 0.001 |
66.7 (53.3–78.3) |
71.7 (58.6–82.5) |
70.2 (56.8–80.9) |
68.3 (55.1–80.1) |
|
Calprotectin > 238 pg/mL |
0.967 (0.922–1.012) |
< 0.001 |
96.7 (88.5–99.6) |
100.0 (94.0–100.0) |
100.0 (93.8–100.0) |
96.8 (88.8–100.0) |
The area under the curve for the CLP value was 0.967: This was statistically significant (
P < 0.001). The value of 238 pg/mL was determined to have 96.7% sensitivity and 100.0% specificity. The positive predictive value was 100.0%, and the negative predictive value was 96.8% (
Table 3). There was no significant difference between hepcidin and CLP values in terms of clinical features and gene mutations (
P > 0.05).
DISCUSSION
FMF is the most common monogenic familial periodic fever syndrome with autosomal recessive inheritance.
11 The Tel-Hashomer criteria are most commonly used for diagnosis. However, diagnosis is very difficult especially in patients with atypical attack episodes. Genetic mutations are quite controversial in diagnosis. In particular, the effect of mutations in exon 2 is not clear. In the long term, chronic inflammation causes various complications—particularly amyloidosis. Subclinical inflammation is a condition that can be also seen in attack-free periods in FMF patients. Although acute phase reactants are the most commonly used parameters for diagnosis, acute phase responses may be insufficient to show subclinical inflammation. It is clear that biomarkers that demonstrate subclinical inflammation are needed.
Hepcidin is a protein that is released from the liver in chronic infections that reduces iron usage and absorption. Serum levels increase in inflammation and infections. Although hepcidin is elevated during inflammation, there are different results in studies related to disease activities. Dağli et al.
12 found a strong correlation between disease activity and hepcidin and pro-hepcidin in patients with RA, and these analytes may play an important role in the pathophysiology of RA-related anemia of chronic disease. Østgård et al.
13 studied RA patients and found that hepcidin levels were lower in healthy subjects versus the pretreatment RA group. They found that hepcidin levels returned to normal after treatment. However, no correlation between hemoglobin levels and disease severity was determined.
In our study, hepcidin levels were determined to be significantly lower in the FMF group than the HC group. The reason why hepcidin levels are not high in the FMF patient group may be that the patients included here were in the attack-free period and under treatment. In addition, even though hemoglobin levels were determined to be lower in the FMF patient group than in the HC group, these levels were not within the range of anemia. Besides, no anemia (chronic disease) was present in this cohort. There was also no correlation between hemoglobin level and serum hepcidin level. Thus, our results suggest that hepcidin cannot be used as a marker for subclinical inflammation.
CLP is a heterodimer with a known role in inflammation and released from activated monocytes and neutrophils. Its role in rheumatic diseases has been studied in RA patients and ankylosing spondylitis.
1415 CLP was found to be high in acute scleroderma and Sjögren's syndrome.
7 It can also be used in the follow-up of treatment response. The CLP level can be measured in the feces or serum.
16 Since the main source of CLP is neutrophil and macrophages, there are studies showing that it is high especially in ‘adult onset still's disease’ with innate immune disease.
17 Here, the CLP level was determined to be significantly higher in the FMF group than the HC group. In parallel with studies in the literature, we found here that CLP can be used as a biomarker for exclusion of FMF diagnosis and disease follow-up—especially due to its high negative predictive value. High levels of CLP even in attack-free periods indicate that there may be a persistent subclinical inflammation or chronic inflammation present in patients with FMF. However, the presence of subclinical inflammation in our FMF patient group is not supported because hepcidin levels are not high, anemia of chronic disease is not observed, and other acute phase reactants are normal. Therefore, our study results suggest that normal/low CLP levels may be used to exclude FMF diagnosis.
The limitations of our study were that it was cross-sectional with no FMF patients in the attack period; there was also a relatively low number of patients. In addition, the measurement of CLP was done in serum and not at the cellular level.
In this study that we compared serum hepcidin and CLP levels in FMF and HC groups. Serum hepcidin levels were lower and CLP levels were higher in FMF patients compared to the HC group. The high CLP levels may show subclinical inflammation in FMF patients. There was no correlation between these biomarkers and MEFV gene mutations. Larger and more comprehensive studies are needed to identify any such correlations. These studies should compare individuals at the time of the attack and attack-free periods with the HCs.









 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download