Abstract
Background
Recently, the number of nationwide medical researches on psoriasis using the National Health Insurance Service database has been on the rise. However, identification of psoriasis using diagnostic codes alone can lead to misclassification. Accuracy of the diagnostic codes and their concordance with medical records should be validated first to identify psoriasis patients correctly.
Objective
To validate the diagnostic codes of psoriasis (International Classification of Diseases, 10th Revision L40) and to find the algorithm for the identification of psoriasis.
Methods
We collected medical records of patients who received their first diagnostic codes of psoriasis during 5 years from five hospitals. Fifteen percent of psoriasis patients were randomly selected from each hospital. We performed a validation by reviewing medical records and compared 5 algorithms to identify the best algorithm.
Results
Total of 538 cases were reviewed and classified as psoriasis (n=368), not psoriasis (n=159), and questionable (n=11). The most accurate algorithm was including patients with ≥1 visits with psoriasis as primary diagnostic codes and prescription of vitamin D derivatives. Its positive predictive value was 96.5% (95% confidence interval [CI], 93.9%~98.1%), which was significantly higher than those of the algorithm, including patients with ≥1 visits with psoriasis as primary diagnostic codes or including ≥1 visits with diagnostic codes of psoriasis (primary or additional) (91.0% and 69.8%). Sensitivity was 90.8% (95% CI, 87.2%~93.4%) and specificity was 92.5% (95% CI, 86.9%~95.9%).
Psoriasis is a chronic immune-mediated inflammatory disorder, which affects approximately 0.5%~3% of the general population1. Recently, we saw a significant increase in the number of psoriasis researches using the nationwide health insurance and claims databases2. Population-based studies can facilitate research on the psoriasis-associated comorbidities, and their psychosocial and economic impact on the patients and produce more useful and qualified results13.
In Korea, two nationwide databases gathered respectively by the National Health Insurance System (NHIS) and the Health Insurance Review and Assessment Service (HIRA) cover nearly 98% of the Korean population. Researches based on insurance claims data identify psoriasis patients usually by using the diagnostic codes45. However, since electronic medical charts are not directly linked with the NHIS and HIRA databases, identifying patients with diagnostic codes alone can result in the misclassification of the concerned patients. According to a report from HIRA, the inconsistency rate between diagnostic codes used for insurance claims and the final diagnosis stated in the electronic medical records (EMR) varies from 23.6% to 61.4%, depending on the diseases6. Therefore, in order to ensure the credibility of any research efforts using these databases, the accuracy of the diagnostic codes and their concordance with the EMR should be validated first.
In countries such as Taiwan7, Sweden8, and Denmark9, which have an established nationwide or provincial healthcare database system, diagnostic code validation tests precede any population-based cohort studies of psoriasis. However, not much effort has been made to verify the validity of psoriasis codes used in cohort studies in Korea.
In this study, we aimed to validate psoriasis' diagnostic code used in insurance claims by comparing them with the final diagnosis in EMR. We also analyzed the validity of several algorithms using diagnostic codes and prescription data to develop the most accurate algorithm for the identification of psoriasis patients in Korea.
This research is a retrospective, multicenter study participated by Inje University of Korea's five affiliated hospitals (four secondary- and one tertiary-level) located in three different cities of Seoul, Goyang, and Busan. It used the diagnostic codes from the International Classification of Diseases, 10th Revision (ICD-10) because both NHIS and HIRA databases are based on these codes. Psoriasis patient data, including their diagnostic codes, age, sex, and prescribed treatments (topical vitamin D derivatives and narrow band ultraviolet B [UVB] phototherapy) was retrieved from each hospital's EMR. Topical vitamin D derivatives includes both single and combination agents with topical steroid.
This study was approved by the institutional review board of Inje University Hospitals: Ilsan Paik hospital (IRB no. 2018-02-010), Busan Paik hospital (IRB no. 2018-0125), Sanggye Paik hospital (IRB no. 2018-07-024), Haeundae Paik hospital (IRB no. 2018-07-005), and Seoul Paik hospital (IRB no. 2018-06-009).
We collected the medical records of outpatients who visited the dermatology department and were assigned a psoriasis code for the first time during 5 years from January 1, 2012 to December 31, 2016. The diagnostic codes which we considered as describing psoriasis were L40.0 (psoriasis vulgaris), L40.1 (generalized pustular psoriasis), L40.2 (acrodermatitis continua), L40.4 (guttate psoriasis), L40.5 (arthropathic psoriasis), L40.8 (other psoriasis) and L40.9 (psoriasis, unspecified) of ICD-10. The diagnostic codes of M07.0 (distal interphalangeal psoriatic arthropathy), M07.1 (arthritis mutilans), M07.2 (psoriatic spondylitis), M07.3 (psoriatic arthropathies) and M09.0 (juvenile arthritis in psoriasis) were also regarded as representing the same disease as the one by L40.5. Patients coded as L40.3 (palmoplantar psoriasis) were excluded as it can be easily confused with other diseases like palmoplantar keratoderma, pompholyx and hand eczema8. We also excluded the inpatient data as well as those from the other departments, including rheumatology.
From each hospital, we randomly sampled 15% of the patients as described in the section above. Then, dermatology specialists of each hospital reviewed the EMR of the sampled patients. After reviewing all the sampled patients' medical records, clinical photos, pathologic reports, and prescribed drugs, we classified them into three groups: psoriasis, not psoriasis, and questionable to diagnose (Fig. 1). We compared positive predictive value (PPV), sensitivity, and specificity of several algorithms to identify psoriasis patients by using diagnostic codes and insurance claim data. We analyzed the type of psoriasis diagnostic code, whether psoriasis code was a primary diagnosis or additional diagnosis in each visit. Furthermore, we counted the number of visits in which the psoriasis was recorded as a primary diagnosis and additional diagnosis, respectively. ICD-10 guidelines divide patients' diagnosis into primary diagnosis and additional diagnosis. Primary diagnosis is defined as the condition primarily responsible for the treatment. Additional diagnosis is the conditions that develop or coexist with primary diagnosis and affects the management of the patients6. We also analyzed the algorithms including the vitamin D derivatives prescription data together with the diagnostic codes, because topical vitamin D derivatives are widely and prescribed specifically for psoriasis than for any other dermatosis. Total of five algorithms were analyzed: 1) ≥1 visits with diagnostic codes of psoriasis (primary or additional); 2) ≥1 visits with psoriasis as a primary diagnostic code; 3) ≥2 visits with psoriasis as a primary diagnostic code; 4) ≥3 visits with psoriasis as a primary diagnostic code; and 5) ≥1 visits with psoriasis as a primary diagnostic code and prescription of vitamin D derivatives, including single and combination agents with topical steroids.
Collected data were analyzed by IBM SPSS Statistics ver. 22.0 (IBM corp., Armonk, NY, USA). A p<0.05 was regarded as statistically significant. PPV, sensitivity, and specificity were estimated with 95% confidence intervals (CI). As PPV is the percentage of patients captured by an algorithm who actually have the disease of interest, the algorithm with higher PPV can more successfully identify whether the patients do or do not have the target disease1011. Additionally, both sensitivity and specificity are concerned as secondary factors to determine a more valuable algorithm12.
The sampled data had a total of 3,587 patients who have either a primary or additional psoriasis diagnostic codes. Randomly selected 538 cases or 15.0% of the whole sample were reviewed. Table 1 shows the characteristics of those sampled patients. The data were comprised of 249 males (46.3%) and 289 females (53.7%). After reviewing the medical record of each patient, we classified 368 patients (68.4%) as psoriasis; 159 (29.6%) as not psoriasis; and 11 (2.0%) as questionable to diagnose. The most commonly used diagnostic code was L40.9 ‘psoriasis, unspecified’ as found in the case of 284 patients (52.8%), followed by L40.0 ‘psoriasis vulgaris’ as in 188 patients (34.9%), and L40.8 ‘other psoriasis’ as in 36 patients (6.7%). A total of 394 patients (73.2%) had psoriasis as a primary diagnostic code, while 144 patients (26.8%) had as additional diagnostic codes. While topical vitamin D derivatives, including single and combination agents, were prescribed to 448 patients (83.3%), only 134 (24.9%) were treated with narrowband UVB phototherapy.
We assessed the PPV, sensitivity, and specificity of each algorithm and compared one another. Since only 11 patients (2.0%) were classified as questionable to diagnose after the medical chart review, they were excluded from this study. The PPV of the algorithm including the patients with ≥1 visits with psoriasis as a primary diagnostic code was much higher than that of the algorithm, including ≥1 visits with diagnostic codes of psoriasis (primary or additional; 91.0% vs. 69.8%) (Table 2).
The validation results of algorithms by the number of visits with psoriasis as a primary diagnostic code are shown in Table 3. Requiring ≥1 visits with psoriasis as a primary diagnostic code revealed a PPV of 91.0% (95% CI, 87.6~93.6) with sensitivity 96.2% (95% CI, 93.6~97.8) and specificity 78.0% (95% CI, 70.6~84.0). While the PPVs and specificity of the algorithms increased in proportion to the number of visits at which psoriasis was a primary diagnostic code, the sensitivity decreased according to the number of visits. The PPV of the algorithm, including patients with ≥3 visits with psoriasis as a primary diagnostic code, was 96.9% (95% CI, 93.3~98.6) and the specificity was 95.6% (95% CI, 90.8~98.1), but the sensitivity was 58.4% (95% CI, 53.2~63.5).
We also analyzed the algorithms, including the prescription of topical vitamin D derivatives as single and combination agents with a topical steroid (Table 4). Algorithm including patients with ≥1 visits with psoriasis as a primary diagnostic code and prescription of vitamin D derivatives showed higher PPV (96.5%, 95% CI, 93.9~98.1) with sensitivity 90.8% (95% CI, 87.2~93.4) and specificity 92.5% (95% CI, 86.9~95.9) than the one including the patients with ≥1 visits with psoriasis as a primary diagnostic code only (91.0%, 95% CI, 87.6~93.6).
Nationwide health insurance databases provide population-based information of patients and healthcare services accumulated over a long period of time13. In South Korea, two nationwide databases gathered respectively by NHIS and HIRA provide population-based health insurance data, which cover almost 98% of the Korean population141516. However, these databases have some limitations. First, as they were not established for medical researches, their structures were not explicitly designed to facilitate the identification of prevalence and incidence of a disease. Second, NHIS and HIRA databases have both primary and additional diagnoses along with the prescriptions and claims data, which makes it further challenging to identify a particular disease16.
Psoriasis researches using health insurance records are increasing around the world. The validation of diagnostic code is very important in the study using this data to reduce the risk of bias. Several countries, including the USA, Sweden, and the UK, performed studies to validate the algorithms to identify psoriasis patients by using their nationwide databases817181920. In the UK, Huerta et al.17 reported that the PPV was 82%, when they selected patients who were referred to a specialist or hospitalized; received psoriasis treatment including topical and/or systemic; or was diagnosed with psoriasis one or more times during their research period. In Sweden, Löfvendahl et al.8 reported that when based on the data from the Skane Healthcare Register, requiring patients with ≥2 diagnostic codes in specialized care could increase the PPV up to 100%. Seminara et al.18 performed a validation study using Read Codes of the UK's Health Improvement Network. They validated an algorithm of ≥2 Read Codes of psoriasis in other dates with high PPV (95%) but lower sensitivity (74%) and specificity (67%).
Several nationwide psoriasis studies used the database of NHIS in Korea21. In these studies, researchers identified psoriasis patients by including those who had at least one diagnostic code of psoriasis without validation. However, identifying psoriasis patients in this way can cause bias because many non-psoriasis patients can be included in the study population4521.
In our study, the algorithm, including ≥1 visits with psoriasis as a primary diagnostic code showed a higher PPV than the one including ≥1 visits with diagnostic codes of psoriasis (primary or additional) (91.0% vs. 69.8%). Generally, additional diagnosis is considered when the conditions develop or coexist with primary diagnosis and all other disease than primary diagnosis. Therefore, the inclusion of the patients who have psoriasis as an additional diagnostic code can raise the possibility of misclassification. The PPV of an algorithm increased proportionally to the frequency of visits with diagnostic codes of psoriasis, as found similarly in the Swedish study8. However, the sensitivity decreased significantly according to the number of visits. Because some patients visited only 1 or 2 times with psoriasis diagnostic code during the study period and some patients' diagnostic code changed from psoriasis to other diseases during follow-up. It can cause a decrease in sensitivity but an increase of specificity.
However, identifying patients with a primary diagnostic code alone still has some potential for misclassification. In other words, even if a patient has received a primary diagnostic code for psoriasis at the initial visit or follow-ups, the patient can still be false-positive if the diagnosis rendered is not final. Icen et al.19 and Ahlehoff et al.22 suggested an algorithm of combining the topical vitamin D derivatives prescription data with the diagnostic codes to improve the validity of the data. Our result also showed that the PPV hit the highest (96.5%) when the algorithm included patients of ≥1 visits with psoriasis as a primary diagnostic code and prescription of topical vitamin D derivatives. However, as our study includes the patients diagnosed as psoriasis by dermatology specialists at secondary or tertiary hospitals only, the prescription rate of vitamin D derivatives can be higher than the cases in which the patients diagnosed by general physicians, rheumatologists, or dermatologists in primary medical care are included. Therefore, the algorithm, including patients with ≥1 visits with psoriasis as a primary diagnostic code, could be an option for the studies, including general physicians and dermatologists in primary medical care, to increase the sensitivity of the algorithm. In our study, the sensitivity of the algorithm, including patients with ≥1 visits with psoriasis as a primary diagnostic code was 96.2%, which was higher than that of the algorithm included the prescription data of topical vitamin D derivatives (90.8%).
Moreover, in our study, patients treated with phototherapy were fewer than we expected (24.9%). Therefore, incorporating phototherapy into the algorithm may result in a significant loss of actual psoriasis patients, especially those with mild symptoms.
Meanwhile, this study should be read with the following limitations in mind. First, confirmation of psoriasis diagnosis was based on the review of medical record, which is subject to the uncertainty and bias of retrospective review. Second, this study used the data from the dermatology department of a general hospital only, which means that the diagnoses rendered by general physicians, rheumatologists, or dermatologists in primary medical care were not considered. Notwithstanding these limitations, however, this study is meaningful in many ways. First, it is the nation's first report on the validity of diagnostic codes used for the identification of psoriasis patients in Korea. Second, as a multi-centered study using the data from different cities of the country, it produced a result neutral to the selection bias and geographical differences.
In conclusion, the algorithm, including patients with ≥1 visits with psoriasis as a primary diagnostic code and prescription of vitamin D derivatives, is the best-validated algorithm to identify psoriasis patients with high PPV (96.5%) and high specificity (92.5%). Furthermore, the algorithm including patients with ≥1 visits with primary diagnostic code of psoriasis, is another option when the study needs higher sensitivity or includes the diagnoses rendered by general physicians, rheumatologists, or dermatologists in primary medical care because it also has relatively high PPV (91.0%) and sensitivity (96.2%). These algorithms can help enhance the credibility of nationwide medical researches on psoriasis, which obtain their data from either NHIS or HIRA database.
References
1. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013; 133:377–385. PMID: 23014338.

2. Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004; 57:131–141. PMID: 15125622.

3. Kim ES, Han K, Kim MK, Park YM, Baek KH, Moon SD, et al. Impact of metabolic status on the incidence of psoriasis: a Korean nationwide cohort study. Sci Rep. 2017; 7:1989. PMID: 28512338.

4. Han JH, Lee JH, Han KD, Seo HM, Bang CH, Park YM, et al. Epidemiology and medication trends in patients with psoriasis: a nationwide population-based cohort study from Korea. Acta Derm Venereol. 2018; 98:396–400. PMID: 29265167.

5. Lee JY, Kang S, Park JS, Jo SJ. Prevalence of psoriasis in Korea: a population-based epidemiological study using the Korean National Health Insurance Database. Ann Dermatol. 2017; 29:761–767. PMID: 29200766.

6. Park BJ, Suh SW, Sung JH, Park GD, Kim SH. Improvement plan for validity of health insurance disease code and establishment of data application plan. Seoul: Health Insurance Review Agency Research Service;2003.
7. Lee MS, Yeh YC, Chang YT, Lai MS. All-cause and cause-specific mortality in patients with psoriasis in Taiwan: a nationwide population-based study. J Invest Dermatol. 2017; 137:1468–1473. PMID: 28257796.
8. Löfvendahl S, Theander E, Svensson Å, Carlsson KS, Englund M, Petersson IF. Validity of diagnostic codes and prevalence of physician-diagnosed psoriasis and psoriatic arthritis in southern Sweden--a population-based register study. PLoS One. 2014; 9:e98024. PMID: 24875275.
9. Ahlehoff O, Gislason GH, Jørgensen CH, Lindhardsen J, Charlot M, Olesen JB, et al. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish nationwide cohort study. Eur Heart J. 2012; 33:2054–2064. PMID: 21840930.

10. Trevethan R. Sensitivity, specificity, and predictive values: foundations, pliabilities, and pitfalls in research and practice. Front Public Health. 2017; 5:307. PMID: 29209603.

11. Parikh R, Mathai A, Parikh S, Chandra Sekhar G, Thomas R. Understanding and using sensitivity, specificity and predictive values. Indian J Ophthalmol. 2008; 56:45–50. PMID: 18158403.

12. Hawass NE. Comparing the sensitivities and specificities of two diagnostic procedures performed on the same group of patients. Br J Radiol. 1997; 70:360–366. PMID: 9166071.

13. Raghupathi W, Raghupathi V. Big data analytics in healthcare: promise and potential. Health Inf Sci Syst. 2014; 2:3. PMID: 25825667.

14. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017; 32:718–728. PMID: 28378543.

15. Kim L, Kim JA, Kim S. A guide for the utilization of Health Insurance Review and Assessment service national patient samples. Epidemiol Health. 2014; 36:e2014008. PMID: 25078381.

16. Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, et al. Data resource profile: the national health information database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017; 46:799–800. PMID: 27794523.

17. Huerta C, Rivero E, Rodríguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007; 143:1559–1565. PMID: 18087008.

18. Seminara NM, Abuabara K, Shin DB, Langan SM, Kimmel SE, Margolis D, et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol. 2011; 164:602–609. PMID: 21073449.

19. Icen M, Crowson CS, McEvoy MT, Gabriel SE, Maradit Kremers H. Potential misclassification of patients with psoriasis in electronic databases. J Am Acad Dermatol. 2008; 59:981–985. PMID: 18835060.

20. Asgari MM, Wu JJ, Gelfand JM, Salman C, Curtis JR, Harrold LR, et al. Validity of diagnostic codes and prevalence of psoriasis and psoriatic arthritis in a managed care population, 1996-2009. Pharmacoepidemiol Drug Saf. 2013; 22:842–849. PMID: 23637091.

21. Oh EH, Ro YS, Kim JE. Epidemiology and cardiovascular comorbidities in patients with psoriasis: a Korean nationwide population-based cohort study. J Dermatol. 2017; 44:621–629. PMID: 28191654.

22. Ahlehoff O, Gislason GH, Charlot M, Jørgensen CH, Lindhardsen J, Olesen JB, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011; 270:147–157. PMID: 21114692.

Fig. 1
Flow chart of study population selection. Total 3,587 patients with psoriasis diagnostic codes were included. Random samples of 538 cases were collected (15% of total cases from each hospital). After the review, 368 (68.4%) were psoriasis, 159 (29.6%) were not psoriasis, and 11 (2.0%) were questionable to diagnosis. ICD: International Classification of Diseases.
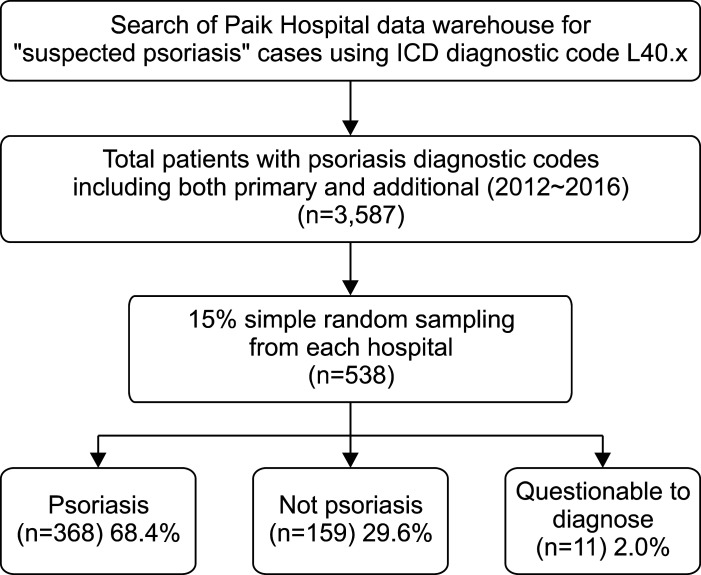
Table 1
Patient demographics and clinical characteristics (n=538)
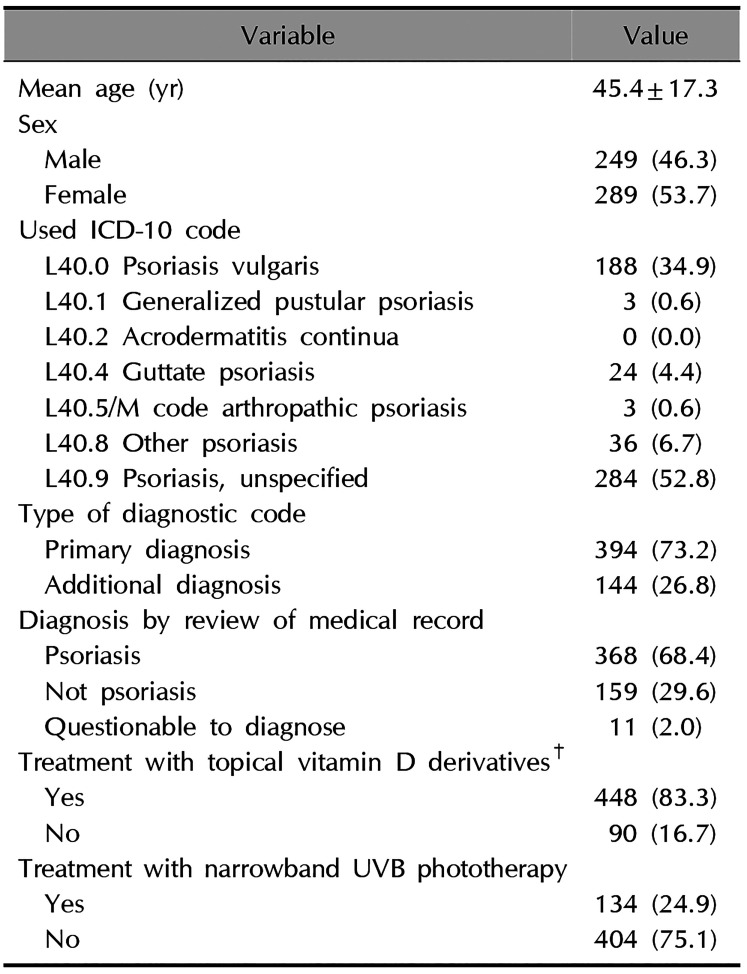
Values are presented as mean±standard deviation or number (%). ICD-10: International Classification of Diseases, 10th Revision, UVB: ultraviolet B. M code: M07.0 Distal interphalangeal psoriatic arthropathy, M07.1 Arthritis mutilans, M07.2 Psoriatic spondylitis, M07.3 Psoriatic arthropathies, M09.0 Juvenile arthritis in psoriasis. †Topical vitamin D derivatives: include single and combination agents with topical steroids.
Table 2
Comparing algorithm of primary diagnostic code with algorithm with both primary and additional diagnostic code

Table 3
Validation of algorithms according to the number of visits with psoriasis as a primary diagnostic code
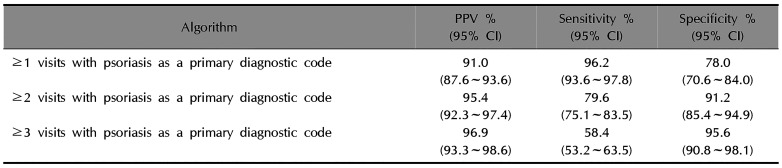
Table 4
Validation and comparison of algorithm including prescription data of topical vitamin D derivatives





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download