Dear Editor:
Various drugs can cause diverse cutaneous adverse drug reactions (CADR)
1. Factors have been implicated in CADR, including the dosage, duration of use, physiological status and genetic background of the patient
1. In addition, current or past viral infection has been reported to affect the occurrence of CADR
2. In particular, many authors have suggested the activation of human herpes virus (HHV) 6, 7, Epstein-Barr virus (EBV), and cytomegalovirus (CMV) in patients with drug-induced hypersensitivity syndrome (DIHS)
23. However, aside from DIHS, there are scarce data regarding the relationship of HHV 6, 7, EBV, and CMV with the overall CADR. Herein, we report viral expression in patients showing various types of CADR in real clinical situations.
Data were analyzed for 26 consecutive patients diagnosed with CADR. The patients provided informed consent for participating in this study and the study was approved by the Institutional Review Board (no. 2016-10-033). The diagnosis was based on history of drug use and clinical features. The presence of HHV 6, 7, EBV, and CMV was confirmed from blood samples and tissue biopsy obtained from the patients 1~2 days after appearance of the typical skin lesion. DNA was extracted using QIAamp DNA mini kit (Qiagen, Hilden, Germany) and paraffin-embedded tissues using the QIAamp® DNA FFPE Tissue kit (Qiagen), and amplified with real-time polymerase chain reaction (PCR) using primers for the EBV R-gene (Argene, Varihes, France) and the CMV, HHV 6, 7, 8R genes (Argene) with oasigTM 2x qPCR Mastermix (Thermofisher, Waltham, MA, USA) and a LightCycler system (Roche, Indianapolis, IN, USA). Serum EBV capsid antigen immunoglobulin M (IgM) and anti-CMV IgM levels were also assessed.
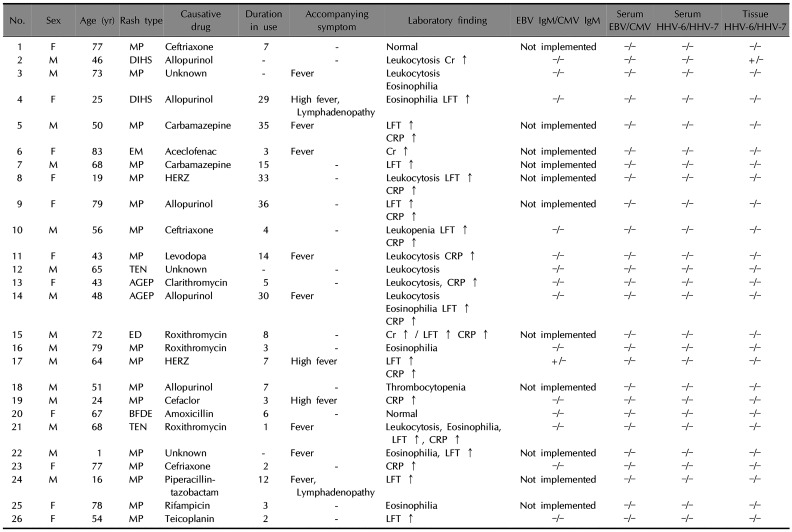
The demographic characteristics and clinical and laboratory findings of the patients are summarized in
Table 1. The most common cutaneous reaction was maculopapular exanthema (MPE), with two cases each of DIHS, acute generalized exanthematous pustulosis (AGEP), and toxic epidermal necrolysis, along with one case each of erythema multiforme, exfoliative dermatitis, and bullous fixed drug eruption. Drug allergy encompasses a spectrum of immunologically-mediated hypersensitivity reactions due to various mechanisms
4. Especially, hypersensitivity reactions to drugs often presents with known immune-mediated reactions including fever, rashes, cytopenia, vasculitis and even anaphylaxis
5. Cutaneous eruption with maculopapular rashes without fever and internal organ damage was diagnosed with MPE, and with fever or internal organ damage was diagnosed with DIHS as in two patients taking allopurinol. Patients with AGEP showed edematous diffuse erythema with multiple, sterile non-follicular pustules
6. The suspected causative agent was antibiotics in 11 cases: three involving ceftriaxone, three involving roxithromycin, and one each involving clarithromycin, cefaclor, amoxicillin, piperalcillin-tazobactam, and teicoplanin. Anti-tuberculosis drugs were suspected in three cases, including isoniazid, rifampin, ethambutol, and pyrazinamide. Other suspected drugs were allopurinol, carbamazepine, aceclofenac, and levodopa. Four patients reported simultaneous use of multiple drugs making it difficult to determine the causative agent. The period from drug administration to skin symptoms ranged from 1 day to 36 days, with an average of 13 days. Real-time PCR demonstrated negative results for HHV 6, 7, EBV, and CMV DNA in the blood samples of all patients. However, the tissue sample of one patient with DIHS was positive for HHV 6. Only one patient was positive for the EBV capsid antigen IgM, who developed a MPE after taking anti-tuberculosis medication; none of the patients was positive for serum anti-CMV IgM.
Overall, we could not find direct evidence of an association of HHV 6, 7, EBV, and CMV with CADR, except for one patient with DIHS. The positive result for serum EBV capsid antigen IgM with negative EBV PCR in only one patient is not enough to conclude that there is a direct association between virus and MPE
7. In previous study that investigated the association between CADR and HHV 6, EBV, and CMV except HHV 7, positive results were observed only in DIHS, but negative results in all MPE cases
2. The reason for the negative findings of almost all of the viral markers in this cohort is unclear. The blood and tissue samples might not have been obtained within an adequate window for detection. Alternatively, this might reflect a weak association between these viruses and overall CADR in contrast to the clear association of viral reactivation in the pathophysiology of DIHS, as suggested by a Japanese consensus group
8. Thus, DIHS might represent a distinct disease entity to other CADR.
In conclusion, there is still no clinical proof of a relationship between reactivation of HHV 6, 7, EBV, and CMV and overall CADR, except DIHS. Inclusion of a larger number of samples and diverse timing of sampling would be needed to confirm our results.
ACKNOWLEDGMENT
This research was supported by the 2016 Bisa Research Grant of Keimyung University. The biological specimens and data used in this study were provided by the Keimyung University Dongsan Hospital Biobank, a member of the Korea Biobank Network.
References
1. Abdullahi Rabiu A, Nordin Bin S, Mainul H. Adverse drug reactions: predisposing factors, modern classifications and causality assessment. Res J Pharm Tech. 2014; 7:1091–1098.
2. Ozcan D, Seçkin D, Bilezikçi B, Arslan H. The role of human herpesvirus-6, Epstein-Barr virus and cytomegalovirus infections in the etiopathogenesis of different types of cutaneous drug reactions. Int J Dermatol. 2010; 49:1250–1254. PMID:
21038542.
3. Draz N, Datta S, Webster DP, Cropley I. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome secondary to antituberculosis drugs and associated with human herpes virus-7 (HHV-7). BMJ Case Rep. 2013; 2013:bcr2013010348.

4. Warrington R, Silviu-Dan F, Wong T. Drug allergy. Allergy Asthma Clin Immunol. 2018; 14(Suppl 2):60. PMID:
30275849.

5. Shepherd GM. Hypersensitivity reactions to drugs: evaluation and management. Mt Sinai J Med. 2003; 70:113–125. PMID:
12634903.
6. Hoetzenecker W, Nägeli M, Mehra ET, Jensen AN, Saulite I, Schmid-Grendelmeier P, et al. Adverse cutaneous drug eruptions: current understanding. Semin Immunopathol. 2016; 38:75–86. PMID:
26553194.

7. De Paschale M, Clerici P. Serological diagnosis of Epstein-Barr virus infection: problems and solutions. World J Virol. 2012; 1:31–43. PMID:
24175209.

8. Shiohara T, Iijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol. 2007; 156:1083–1084. PMID:
17381452.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download