Abstract
Solitary fibrous tumors (SFT) are uncommon mesenchymal tumors. SFT have several synonyms including localized fibrous tumor, benign mesothelioma, localized fibrous mesothelioma, and submesothelial fibroma. SFT usually occur in the pleura or other serosal surfaces, but SFT can also develop in extrapleural areas including the nasal cavity, orbit, retroperitoneum, and pelvis. Cutaneous SFT is extremely rare, and more likely to occur in the head and neck region. Histologically, this tumor can mimic a variety of benign and malignant tumors such as dermatofibroma, dermatofibrosarcoma protuberans, spindle cell lipoma or other mesenchymal tumors. Most cases of SFT show non-aggressive clinical courses, with low recurrence rates. Herein, we describe a case of primary cutaneous SFT which presented with huge mass on the back.
Solitary fibrous tumors (SFT) is a rare tumor of mesenchymal origin and most commonly involves the pleura1. However, the presence of SFT has been reported rarely in various parts of body. It is usually located in the liver, kidney, thyroid, nervous system or skin if it develops outside of thoracic cavity. It is known that the SFT that develops primarily in the skin is clinically similar to the cyst or lipoma and appears as a nonspecific single nodule or subcutaneous mass2. Histologically, SFT may be difficult to diagnose because they show various histopathologic features and are characterized by solid spindle cell, diffuse sclerosing, fascicular, storiform, herringbone, angiofibromatous pattern, and so-called ‘patternless pattern’3. Therefore, it is necessary to differentiate from various tumors such as dermatofibroma, dermatofibrosarcoma protuberans, hemangiopericytoma, myofibroma, and spindle cell lipoma1.
We experienced a rare case of SFT presented with a large subcutaneous mass on the skin.
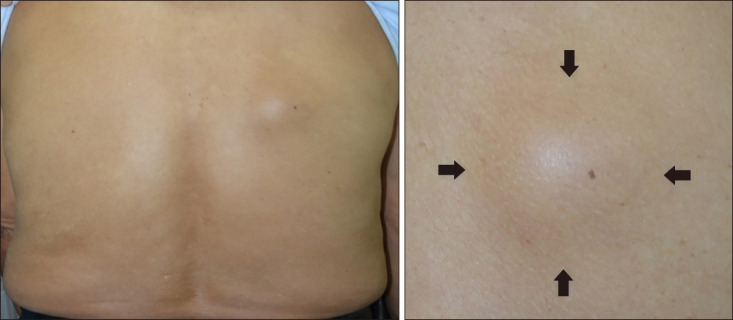
A 74-year-old male presented with slowly growing subcutaneous mass on his upper back for 10 years. He did not complain for pain or other subjective symptoms. Physical examination revealed a hemispheric subcutaneous mass with a diameter of about 5 cm (Fig. 1). It palpated as a solid, mobile mass. There was no tenderness at the time of presentation. On histologic examination, epidermis and dermis showed no specific findings. Solid mass without encapsulation was found on subcutaneous layer (Fig. 2). Fibrous matrix was observed inside of tumor. There were a part of relatively dense cells and a part consisted of cells with low cellularity inside the fibrous matrix. Proliferation of the blood vessels was observed. At high magnification view, spindle cells were composed of “patternless” swirling patterns and showed some spiral patterns. Area with less cellularity was mainly consisted of hyalinized collagen fibers. Irregularly extended blood vessels with thin walls and some staghorn-shaped vessels were found (Fig. 2). The spindle cells had a pale-coloredvesicular nucleus and no cellular dysplasia or mitosis was observed. Immunohistochemical stains revealed positivity for CD34, Bcl-2, CD99, and factor XIIIa. Tumor cells did not stained for smooth muscle actin (SMA) and S-100 (Fig. 3). Based on the histologic findings of the excision biopsy specimen, SFT of the skin was diagnosed. The tumor didn't recur after one year following-up. We received the patient's consent form about publishing all photographic materials.
SFT is a relatively rare mesenchymal tumor that has been described for the first time as a neoplasm composed of spindle-shaped cells in the pleura by Klemperer and Rabin4.
However, it is very rare that a SFT primarily occurs in the skin. Okamura et al.5 reported the first case of SFT on skin in 1997. According to a study of 17 cases of SFT on the skin, asymptomatic subcutaneous masses similar to cysts or lipomas were usually found on head and neck6. Histologically, it is composed of spindle-shaped cells and it is observed that the pattern of hemangiopericytoma-like appearance, spiral pattern, and fibrous spindle cell proliferation are expressed as ‘patternless pattern’7. It is also characterized by alternating areas of high and low cell density8. In immunohistochemical staining, CD34 is mostly positive but it is not a specific finding. Bcl-2, CD99, factor XIIIa staining may be helpful in diagnosis (Table 1)7. Generally, the SFT is benign but local recurrence may occur and periodic follow-up is required9. In some cases, malignant transformation may be seen. There are some opinions to categorize SFT as borderline tumors1. In cases of tumor size greater than 5 cm, infiltrative growth, high cellularity, polymorphism, necrosis, and mitosis more than 4~10 per high power field occur, malignancy can be suspected1.
In this case, SMA stain was negative so myofibroma could be excluded. Myofibroma shows an arrangement of interlacing fascicles of spindle-shaped cells resembling myofibroblasts10. Spindled cells express vimentin and SMA and are usually negative for desmin10. Negative findings on S-100 could exclude tumors of neural origin such as schwannomas. CD34 staining was generally positive, but the demarcation of tumor was fairly good. And histologic findings were more various than those of spindle-shaped cells with uniform morphology. Therefore, it was possible to distinguish the dermatofibrosarcoma protuberance. In addition, Bcl-2, factor XIIIa and CD99 immunohistochemical staining showed positive findings, which is consistent with SFT. We report a rare case of primary SFT on back that was diagnosed by excisional biopsy, showing unusual clinical features.
References
1. Erdag G, Qureshi HS, Patterson JW, Wick MR. Solitary fibrous tumors of the skin: a clinicopathologic study of 10 cases and review of the literature. J Cutan Pathol. 2007; 34:844–850. PMID: 17944724.

2. Kang TW, Kim HJ, Kim YC, Kim SC. A case of solitary fibrous tumor that developed on the scalp. Korean J Dermatol. 2009; 47:615–617.
3. Moran CA, Suster S, Koss MN. The spectrum of histologic growth patterns in benign and malignant fibrous tumors of the pleura. Semin Diagn Pathol. 1992; 9:169–180. PMID: 1609159.
4. Klemperer P, Rabin CB. Primary neoplasms of the pleura: a report of five cases. Arch Pathol. 1931; 11:385–412.

5. Okamura JM, Barr RJ, Battifora H. Solitary fibrous tumor of the skin. Am J Dermatopathol. 1997; 19:515–518. PMID: 9335244.

6. Soldano AC, Meehan SA. Cutaneous solitary fibrous tumor: a report of 2 cases and review of the literature. Am J Dermatopathol. 2008; 30:54–58. PMID: 18212546.

7. Terada T. Solitary fibrous tumor of the shoulder showing diverse distinct histologic patterns. Int J Dermatol. 2011; 50:208–211. PMID: 21244389.

8. Ali SZ, Hoon V, Hoda S, Heelan R, Zakowski MF. Solitary fibrous tumor. A cytologic-histologic study with clinical, radiologic, and immunohistochemical correlations. Cancer. 1997; 81:116–121. PMID: 9126139.
9. Omori Y, Saeki H, Ito K, Matsuzaki H, Tokita M, Itoh M, et al. Solitary fibrous tumour of the scalp. Clin Exp Dermatol. 2014; 39:539–541. PMID: 24712870.

10. Kye H, Kwon IH, Seo SH, Ahn HH, Kye YC, Choi JE. Adult multiple myofibromas on an atrophic patch on the thigh. Ann Dermatol. 2015; 27:622–623. PMID: 26512183.

Fig. 2
(A) Cut section of the tumor showed an ovoid, well defined white-tan solid mass measuring 50×35×28 mm in size. (B) A spheroid, well-circumscribed tumor composed of alternating hypercellular and fibrous hypocellular areas was observed in the subcutis (H&E, ×40). (C) In the highly cellular areas, spindle-shaped cells were present in short interlacing fascicles, mixed with interstitial fibrous tissue (H&E, ×100). (D) In hypocellular foci, interspersed collagen fibers were mainly seen (H&E, ×100). (E) Many of the cells had enlarged vesicular nuclei with inconspicuous nucleoli (H&E, ×400). (F) Staghorn and ectatic blood vessels were found in some areas (H&E, ×200).
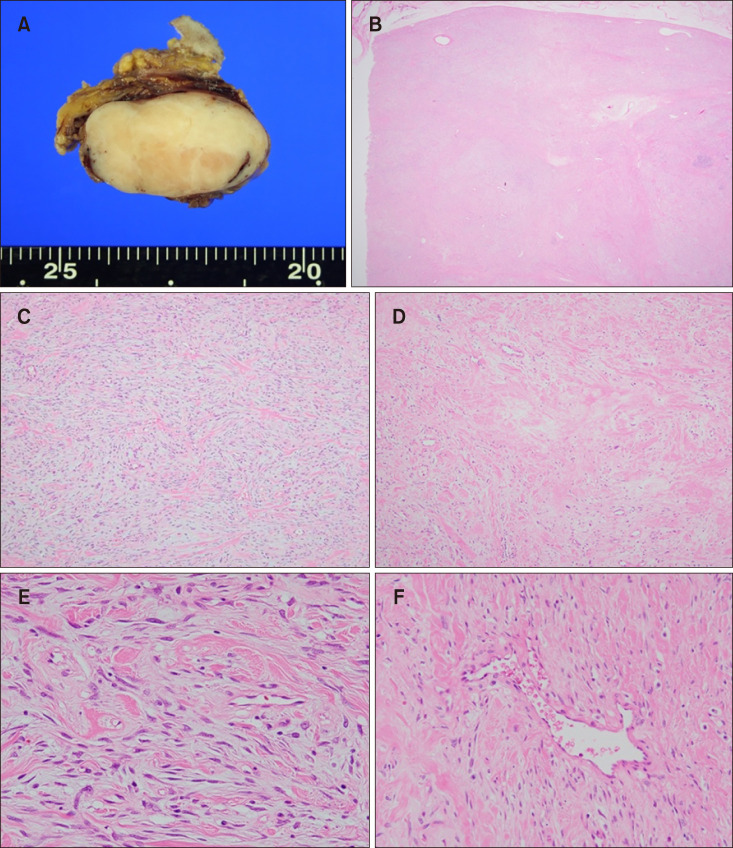
Fig. 3
Immunohistochemical staining was performed for smooth muscle actin (SMA), S-100, CD34, Bcl-2, CD99 and factor XIIIa. The tumor cells demonstrated positivity for CD34, factor XIIIa, CD99 and Bcl-2 (A: CD34, ×200; B: factor XIIIa, ×100; C: CD99, ×100; D: Bcl-2, ×100). However, S-100 and SMA staining were negative in tumor cell (E: S-100, ×100; F: SMA, ×100).
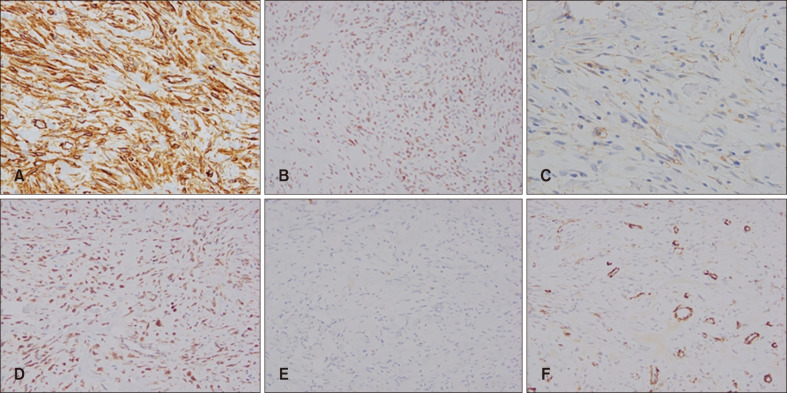
Table 1
Clinicopathologic features of previously reported cases of primary cutaneous SFTs
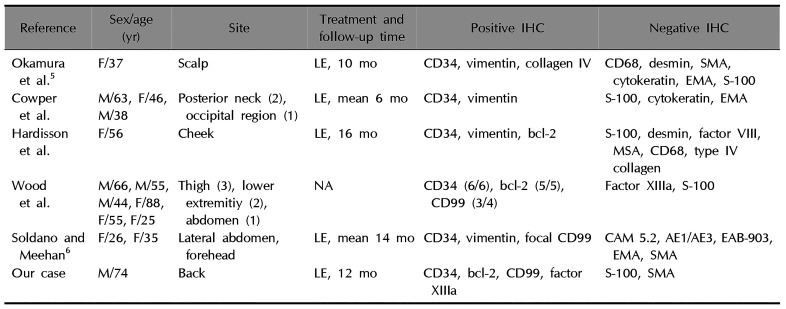
| Reference | Sex/age (yr) | Site | Treatment and follow-up time | Positive IHC | Negative IHC |
|---|---|---|---|---|---|
| Okamura et al5 | F/37 | Scalp | LE, 10 mo | CD34, vimentin, collagen IV | CD68, desmin, SMA, cytokeratin, EMA, S-100 |
| Cowper et al. | M/63, F/46, M/38 | Posterior neck (2), occipital region (1) | LE, mean 6 mo | CD34, vimentin | S-100, cytokeratin, EMA |
| Hardisson et al. | F/56 | Cheek | LE, 16 mo | CD34, vimentin, bcl-2 | S-100, desmin, factor VIII, MSA, CD68, type IV collagen |
| Wood et al. | M/66, M/55, M/44, F/88, F/55, F/25 | Thigh (3), lower | NA | CD34 (6/6), bcl-2 (5/5), | Factor XIIIa, S-100 |
| Soldano and Meehan6 | F/26, F/35 | Lateral abdomen, forehead | LE, mean 14 mo | CD34, vimentin, focal CD99 | CAM 5.2, AE1/AE3, EAB-903, EMA, SMA |
| Our case | M/74 | Back | LE, 12 mo | CD34, bcl-2, CD99, factor XIIIa | S-100, SMA |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download