Abstract
Solitary fibrous tumor (SFT) is a relatively uncommon mesenchymal neoplasm that usually arises in the pleura, but also has been reported in numerous extrapleural locations, including cutaneous site. The skin lesion presents as a circumscribed nodule or tumor, mainly on the head and neck. A 41-year-old male presented with 6 months history of nail lesion without symptom on the left third finger. The lesion is slightly yellowish discoloration with subungual erythematous nodule and distal onycholysis. Biopsy specimen from the nail lesion showed the spindle cells form patternless pattern with hypercellular and hypocellular area. And small blood vessels and dilated vascular spaces were present. The result of special stain for specimen showed that positive for CD34, Bcl-2, and CD99 but negative for S-100, FactorXIIIa, and smooth muscle action. Recognition of this uncommon location of SFT is important because of possible confusion with other subungual tumors, including glomus tumor, fibroma and other fibrohistiocytic tumors like dermatofibrosarcoma protuberans, superficial acral fibromyxoma and cellular digital fibroma. Here in, we report a case of SFT of subungual region. We think this case is interesting because of uncommon location and may be helpful to more understand the character of this disease.
A solitary fibrous tumor (SFT) is a relatively uncommon mesenchymal neoplasm that usually arises in the pleura. While SFTs have been reported to affect various locations, cutaneous SFT has rarely been reported123. SFTs that clinically involve the skin present as a circumscribed nodule, and most commonly occur in the head and neck. We experienced a case of an STF in the subungual region, which was diagnosed using histopathologic examination. Here, we describe this rare case and provide a literature review.
A 41-year-old male patient visited our hospital with the main complaint of an asymptomatic skin lesion beneath the third left nail of the hand. Six months prior, the patient presented with discoloration on the third left nail, a unilateral lesion beneath the nail, and a growing skin lesion without symptoms. There was no relevant history or family history, and there were no abnormalities in the laboratory findings. The patient's lesion was observed in the third left finger with yellowish discoloration and hypertrophy of the nail accompanied by distal onycholysis and an erythematous nodule on the proximal nail fold (Fig. 1). A punch biopsy was performed under the nail. The biopsy results indicated the presence of several small and dilated blood vessels between irregularly arranged proliferated spindle cells, a hypercellular area with several spindle cells, and a hypocellular area with several collagen fibers. Immunohistochemical staining was performed, which showed that the tumor cells were positive for CD34, Bcl-2, and CD99 and negative for S-100, FactorXIIIa, and smooth muscle action (SMA) (Fig. 2). Based on the clinical features and histologic findings, the patient was diagnosed with a subungual SFT. The patient was transferred to the department of plastic surgery for surgical treatment. We received the patient's consent form about publishing all photographic materials.
A SFT is an uncommon neoplasm with a mesenchymal origin that usually arises in the pleura, but is known to occur in other locations, such as the liver, kidney, soft tissue, thyroid, central nervous system, and adrenal glands. Cases of SFT involving the skin are uncommon, but a few have been reported23.
Clinically cutaneous SFTs was first reported by Okamura et al.3. The common cutaneous involvement sites include the head and neck, and cases of SFT development in the trunk and limbs have also been reported4. No SFT of the subungual region has been reported anywhere before, and only 10 cases of SFT in atypical skin areas (except head and neck) have been reported in the literatures (Table 1)145678910111213. The analysis of those cases showed that there was no difference in the incidence of SFT development between men and women. Furthermore, SFTs can develop in individuals in their 20's to 60's and vary in size from 1 to 8 cm. Meanwhile, most patients with an SFT experience either no symptoms or localized tenderness. The potential sites of lesion development include the back, torso, arms, and legs.
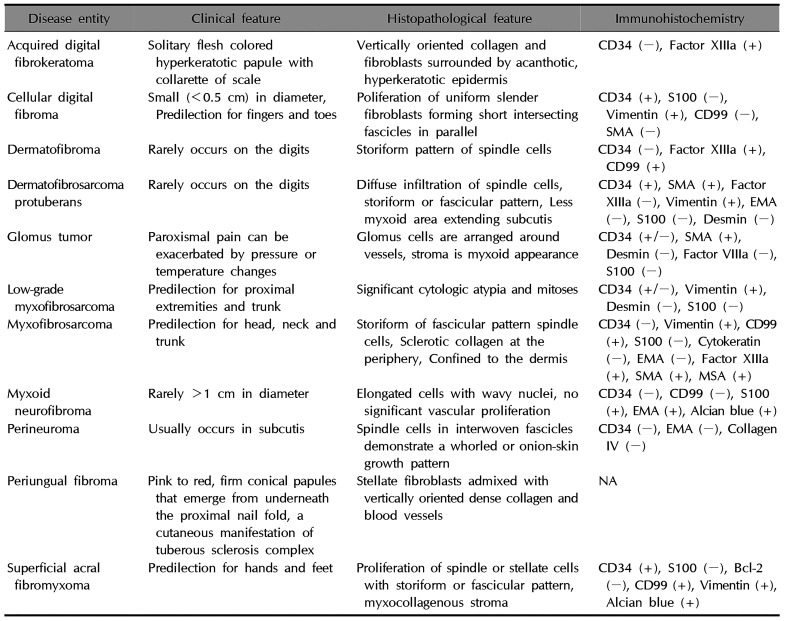
Histologically, SFTs present as proliferating spindle cells that are similar to fibroblasts and are characterized by a “patternless pattern,” as they may exhibit various forms without a specific arrangement. These various forms include a herringbone form, storiform, wavy form, and hemangiopericytoma-like angioplasia form. A SFT may appear histologically similar to dermatofibroma, spindle cell lipoma, leiomyoma, dermatofibrosarcoma protuberans, superficial acral fibromyxoma, cellular digital fibroma, dermatomyofibroma, schwannoma, and hemangiopericytoma, which may include proliferating cells3. Therefore, immunohistochemical staining is helpful in diagnosing an SFT. In the literature, the immunohistochemical findings indicate a variation in the positive detection of CD34, CD99, vimentin, and Bcl-2 in SFTs and an absence of muscle, neural and epithelial markers1.
Genearlly, the CD34 is widely used as marker for differentiating spindle cell pattern tumor. Human CD34 molecule has been originated form myeloid leukemia cell line and used as a marker for hematopoietic progenitor cells. It is also expressed in nonhematopoietic cell types including muscle satellite cells, corneal keratocytes, interstitial cells, epithelial progenitors, and vascular endothelial progenitors. In literature reviews to date, there is no studies reported that CD34 expression imply specific origin. Also there are no reports that SFT is thought to have specific origin. In 2014, Sidney et al.14 reported that the CD34 expression associates with progenitor and stem cell activity and correlates with cell plasticity. Therefore, considering the facts SFTs show diffuse CD34 expression and arise from various body sites, we believe that the SFTs have progenitor activity in mesenchymal stem cells rather than have specific differentiated origin.
In the present case, we could distinguish the patient's SFT from a muscle origin tumor because the immunohistochemical analysis showed a positive staining for CD34 and a negative staining for SMA. Neurofibromatosis and fibrous histiocytoma could be excluded from positive staining results for S-100. In addition, dermatofibrosarcoma protuberans, in which spindle cells are observed, could be excluded because it is characterized by storiform or a fascicular pattern, and is negative for Bcl-2 and CD99. Superficial acral fibromyxoma shows a spindle cell with a storiform or fascicular pattern and negative findings on Bcl-2 immunohistochemical stain. Also this case did not show myxoid change. And cellular digital fibroma is less than 0.5 cm in size and has clinical features similar to viral warts. Histologically, proliferation of uniform slender fibroblasts is seen in the fascicles of the fascicles. Based on the aforementioned clinical features and immunohistochemical staining results, this patient was diagnosed with an SFT of the subungual region (Table 2)1516. Recently, Doyle et al.17 have reported that presence of immunohistochemical stain for signal transductor and activator of transcription 6 (STAT6) and NGFI-A binding protein 2 (NAB2, EGR1 binding protein 2)-STAT6 fusion gene is a highly sensitive and specific markers for SFT. It is limitation that this case not identified the STAT6 immunohistochemical stain or NAB2-STAT6 fusion gene.
Patients with a SFT are usually treated with complete surgical excision. However, no published consent about procedures in the treatment of SFT exists18. The rate of local recurrence or distant metastasis or both is 10%~13%18. Nielsen et al.19 reported that SFT in the extremities are more likely to be malignant. Thus, a wider resection of the tumor and a long term follow-up was proposed. Malignant tumors generally display histological features including hypercellularity, moderate to severe nuclear atypia, stromal or vascular invasion, necrosis and high mitotic activity (over 4 per 10 high-power fields)19.
To our knowledge, there have been no cases reported occurred in the subungual area. Considering this case report, SFT should be considered in differential diagnosis of soft tissue tumor in subungual area. However, differential diagnosis of acral lesion tumor is broad. And it is difficult to distinguish tumors with similar clinical features and histologically showing spindle cells. Therefore, histologic findings, including immunochemical study, are indispensable to differentiate tumors of other acral lesions including SFT. We report this case as a rare educational case.
References
1. Soldano AC, Meehan SA. Cutaneous solitary fibrous tumor: a report of 2 cases and review of the literature. Am J Dermatopathol. 2008; 30:54–58. PMID: 18212546.

2. Erdag G, Qureshi HS, Patterson JW, Wick MR. Solitary fibrous tumors of the skin: a clinicopathologic study of 10 cases and review of the literature. J Cutan Pathol. 2007; 34:844–850. PMID: 17944724.

3. Okamura JM, Barr RJ, Battifora H. Solitary fibrous tumor of the skin. Am J Dermatopathol. 1997; 19:515–518. PMID: 9335244.

4. Oh YJ, Lew BL, Sim WY. Primary cutaneous solitary fibrous tumor. Korean J Dermatol. 2011; 49:1093–1097.
5. Chang SE, Bae GY, Choi JH, Sung KJ, Moon KC, Koh JK, et al. Cutaneous solitary fibrous tumour with myxoid stroma. Br J Dermatol. 2002; 147:1267–1269. PMID: 12452886.

6. Lee HJ, Lee SH, Roh MR. Cutaneous myxoid solitary fibrous tumour. Clin Exp Dermatol. 2012; 37:197–199. PMID: 22092338.

7. Suster S, Nascimento AG, Miettinen M, Sickel JZ, Moran CA. Solitary fibrous tumors of soft tissue. A clinicopathologic and immunohistochemical study of 12 cases. Am J Surg Pathol. 1995; 19:1257–1266. PMID: 7573687.
8. Morgan MB, Smoller BR. Solitary fibrous tumors are immunophenotypically distinct from mesothelioma(s). J Cutan Pathol. 2000; 27:451–454. PMID: 11028815.

9. Yoshida Y, Kubota Y, Yamaguchi T, Iwasaki H, Nakayama J. Subcutaneous solitary fibrous tumor. J Dermatol. 2004; 31:1018–1022. PMID: 15801268.

10. Terada T. Solitary fibrous tumor of the shoulder showing diverse distinct histologic patterns. Int J Dermatol. 2011; 50:208–211. PMID: 21244389.

11. Hata H, Natsuga K, Aoyagi S, Homma E, Shimizu H. Solitary fibrous tumour fluctuating in size with menstrual cycle. Clin Exp Dermatol. 2014; 39:753–755. PMID: 24986672.

12. Lee JY, Park SE, Shin SJ, Kim CW, Kim SS, Kim KH. Solitary fibrous tumor with myxoid stromal change. Am J Dermatopathol. 2015; 37:570–573. PMID: 25140663.

13. Lee JY, Kim DH, Seo KJ, Jung SN. A solitary fibrous tumor (cellular form) of the ankle. J Foot Ankle Surg. 2016; 55:829–831. PMID: 25979291.

14. Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014; 32:1380–1389. PMID: 24497003.

15. Sawaya JL, Khachemoune A. Superficial acral fibromyxoma. Int J Dermatol. 2015; 54:499–508. PMID: 25772615.

16. Lee JY, Park SE, Shin SJ, Kim CW, Kim SS. Diagnostic pitfalls of differentiating cellular digital fibroma from superficial acral fibromyxoma. Ann Dermatol. 2015; 27:462–464. PMID: 26273171.

17. Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014; 27:390–395. PMID: 24030747.

18. Anders JO, Aurich M, Lang T, Wagner A. Solitary fibrous tumor in the thigh: review of the literature. J Cancer Res Clin Oncol. 2006; 132:69–75. PMID: 16283380.

19. Nielsen GP, O'Connell JX, Dickersin GR, Rosenberg AE. Solitary fibrous tumor of soft tissue: a report of 15 cases, including 5 malignant examples with light microscopic, immunohistochemical, and ultrastructural data. Mod Pathol. 1997; 10:1028–1037. PMID: 9346183.
Fig. 1
Slightly yellowish discoloration with hypertrophic nail and distal onycholysis and erythematous subungual nodule on the proximal nail fold of left third finger.
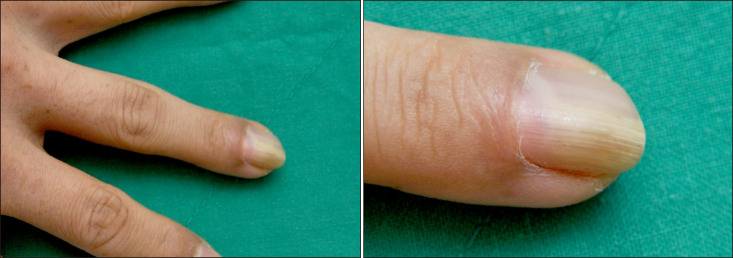
Fig. 2
(A) The biopsy specimen shows proliferation of spindle cells and several small blood vessels and dilated vascular spaces are present (H&E, ×100). (B) The spindle cells form patternless pattern with hypercellular areas (H&E, ×200). (C) The spindle cells form patternless pattern with hypocellular areas (H&E, ×200). (D) The tumor cells are diffusely positive for CD34 (×400). (E) The tumor cells are positive for Bcl-2 (×400). (F) The tumor cells are diffusely positive for CD99 (×400). (G) The tumor cells are negative for S-100 (×400). (H) The tumor cells are negative for FactorXIIIa (×400). (I) The tumor cells are negative for SMA (×400).
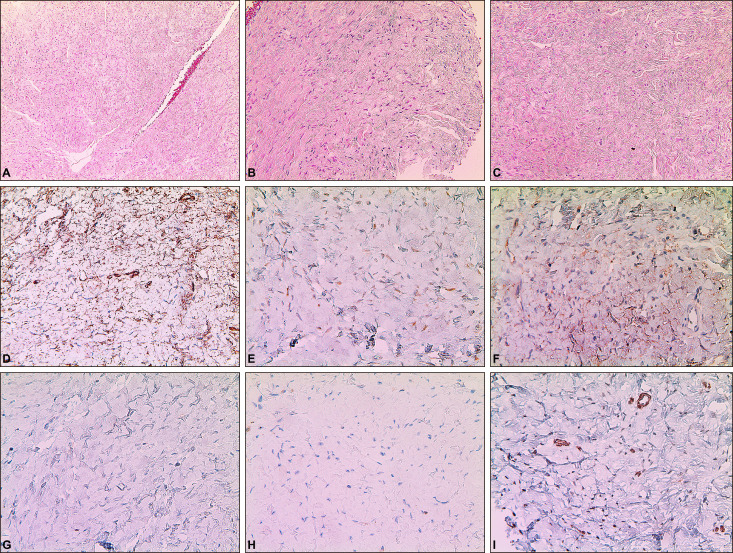
Table 1
Previous reported cutaneous solitary fibrous tumors (except head and neck) in literatures
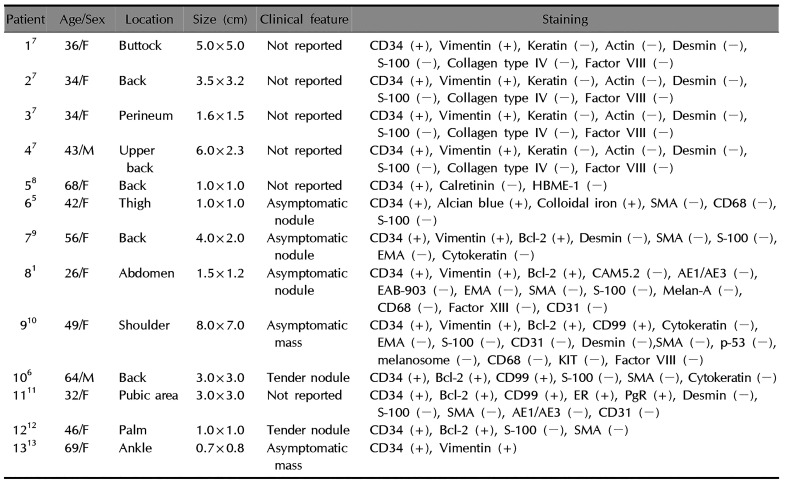
| Patient | Age/Sex | Location | Size (cm) | Clinical feature | Staining |
|---|---|---|---|---|---|
| 17 | 36/F | Buttock | 5.0×5.0 | Not reported | CD34 (+), Vimentin (+), Keratin (−), Actin (−), Desmin (−), S-100 (−), Collagen type IV (−), Factor VIII (−) |
| 27 | 34/F | Back | 3.5×3.2 | Not reported | CD34 (+), Vimentin (+), Keratin (−), Actin (−), Desmin (−), S-100 (−), Collagen type IV (−), Factor VIII (−) |
| 37 | 34/F | Perineum | 1.6×1.5 | Not reported | CD34 (+), Vimentin (+), Keratin (−), Actin (−), Desmin (−), S-100 (−), Collagen type IV (−), Factor VIII (−) |
| 47 | 43/M | Upper back | 6.0×2.3 | Not reported | CD34 (+), Vimentin (+), Keratin (−), Actin (−), Desmin (−), S-100 (−), Collagen type IV (−), Factor VIII (−) |
| 58 | 68/F | Back | 1.0×1.0 | Not reported | CD34 (+), Calretinin (−), HBME-1 (−) |
| 65 | 42/F | Thigh | 1.0×1.0 | Asymptomatic nodule | CD34 (+), Alcian blue (+), Colloidal iron (+), SMA (−), CD68 (−), S-100 (−) |
| 79 | 56/F | Back | 4.0×2.0 | Asymptomatic nodule | CD34 (+), Vimentin (+), Bcl-2 (+), Desmin (−), SMA (−), S-100 (−), EMA (−), Cytokeratin (−) |
| 81 | 26/F | Abdomen | 1.5×1.2 | Asymptomatic nodule | CD34 (+), Vimentin (+), Bcl-2 (+), CAM5.2 (-), AE1/AE3 (-), EAB-903 (-), EMA (-), SMA (-), S-100 (-), Melan-A (-), CD68 (-), Factor XIII (-), CD31 (-) |
| 910 | 49/F | Shoulder | 8.0×7.0 | Asymptomatic mass | CD34 (+), Vimentin (+), Bcl-2 (+), CD99 (+), Cytokeratin (−), EMA (−), S-100 (−), CD31 (−), Desmin (−),SMA (−), p-53 (−), melanosome (−), CD68 (−), KIT (−), Factor VIII (−) |
| 106 | 64/M | Back | 3.0×3.0 | Tender nodule | CD34 (+), Bcl-2 (+), CD99 (+), S-100 (−), SMA (−), Cytokeratin (−) |
| 1111 | 32/F | Pubic area | 3.0×3.0 | Not reported | CD34 (+), Bcl-2 (+), CD99 (+), ER (+), PgR (+), Desmin (−), S-100 (−), SMA (−), AE1/AE3 (−), CD31 (−) 1212 |
| 1212 | 46/F | Palm | 1.0×1.0 | Tender nodule | CD34 (+), Bcl-2 (+), S-100 (−), SMA (−) |
| 1313 | 69/F | Ankle | 0.7×0.8 | Asymptomatic mass | CD34 (+), Vimentin (+) |
Table 2




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download