INTRODUCTION
Tinea capitis is an infectious skin disease caused by dermatophytes that is found primarily in children
1 and more commonly in males. Its clinical manifestation can range from small scaly patches to large inflammatory pustular plaques with extensive hair loss. Immunosuppressive conditions, such as diabetes mellitus and prolonged steroid medication, have also been associated with tinea capitis
2. The epidemiological and mycological characteristics of tinea capitis change over time and are influenced by environmental conditions, socioeconomic status, and individual immunity.
Microsporum ferrugineum was the most common dermatophyte responsible for tinea capitis in Korea in the 1960s
3, but
Microsporum canis has increasingly been identified as the cause of tinea capitis in Korea since the 1970s
4. Although tinea capitis can be controlled with oral antifungal medication and public health campaigns, it remains a public health issue in Korea
5. In particular, the incidence of tinea capitis is increasing in adults, even though it is decreasing in children. We thus investigated changes in the epidemiological and mycological characteristics of adult patients with tinea capitis in southeastern Korea.
Go to :

RESULTS
Annual incidence
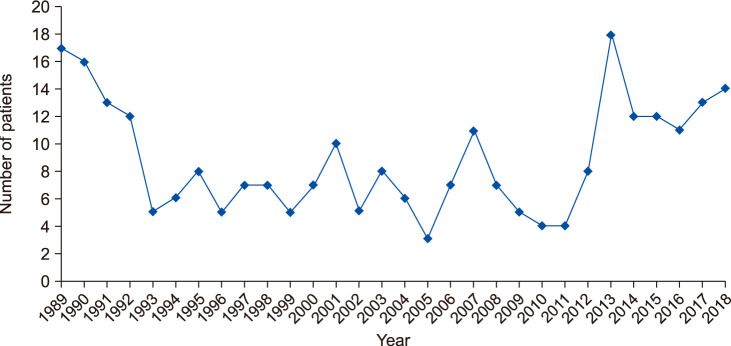
The annual incidence of patients aged over 20 years with tinea capitis ranged from 3 to 18 between 1989 and 2018 (
Fig. 1), with a mean annual incidence rate of 8.9. In terms of the change in the mean incidence rate over time, it was 9.6 between 1989 and 1998 (96 patients), 6.9 between 1999 and 2008 (69 patients), and 10.1 between 2009 and 2018 (101 patients). The annual incidence of tinea capitis in patients aged over 20 years was lowest in 2005 and highest in 2013.
Fig. 2 presents the proportion of tinea capitis patients that were over 20 years old; of the total of 780 patients diagnosed with tinea capitis between 1989 and 1998, 96 were older than 20 (12.31%), compared to 69 out of 268 between 1999 and 2008 (25.75%), and 101 out of 206 between 2009 and 2018 (49.03%) for a total of 266 out of 1,254 for the entire study period (21.21%).
 | Fig. 1Annual incidence for adult patients with tinea capitis.
|
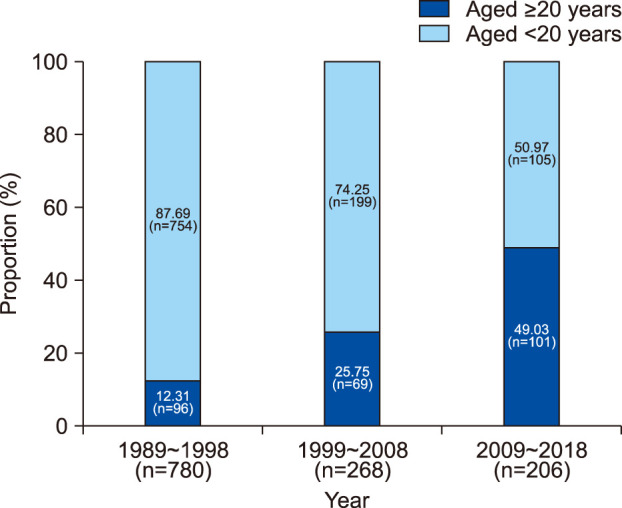 | Fig. 2Proportion of adult patients in total tinea capitis incidence by time period. The proportion of tinea capitis patients aged over 20 years has been increasing with time: 12.31% between 1989 and 1998, 25.75% between 1999 and 2008, and 49.03% between 2009 and 2018.
|
Age distribution
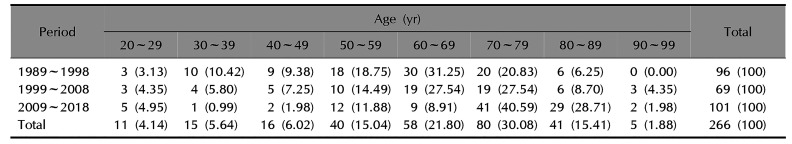
Table 1 presents the incidence of tinea capitis by age group. From 1989 to 2018, the most commonly affected age group were those patients in their seventies, with 80 patients diagnosed (30.08%). Of the remaining patients, 58 (21.80%) were in their sixties, and 41 (15.41%) were in their eighties. The rest were in their fifties, forties, thirties, twenties, and nineties in order. This pattern was similar for the individual 1989~1998, 1999~2008, and 2009~2018 subperiods, though some differences were observed. From 1989 to 1998, the most commonly affected age group was those patients in their sixties, with 30 patients diagnosed (31.25%). Of the remaining patients, 20 (20.83%) were in their seventies, 18 (18.75%) were in their fifties, and the rest were in thirties, forties, eighties, and twenties in order. From 1999 to 2008, 19 patients (27.54%) were respectively in their sixties and seventies, 10 (14.49%) were in their fifties. The rest were in their eighties, forties, twenties, thirties, and nineties in order, each with 6 patients or fewer. From 2009 to 2018, 41 patients (40.59%) were in their seventies, 29 (28.71%) were in their eighties, 12 (11.88%) were in their fifties, and the rest were in their sixties, twenties, forties, nineties, and thirties in order.
Table 1
Age distribution of adult patients with tinea capitis
|
Period |
Age (yr) |
Total |
|
20~29 |
30~39 |
40~49 |
50~59 |
60~69 |
70~79 |
80~89 |
90~99 |
|
1989~1998 |
3 (3.13) |
10 (10.42) |
9 (9.38) |
18 (18.75) |
30 (31.25) |
20 (20.83) |
6 (6.25) |
0 (0.00) |
96 (100) |
|
1999~2008 |
3 (4.35) |
4 (5.80) |
5 (7.25) |
10 (14.49) |
19 (27.54) |
19 (27.54) |
6 (8.70) |
3 (4.35) |
69 (100) |
|
2009~2018 |
5 (4.95) |
1 (0.99) |
2 (1.98) |
12 (11.88) |
9 (8.91) |
41 (40.59) |
29 (28.71) |
2 (1.98) |
101 (100) |
|
Total |
11 (4.14) |
15 (5.64) |
16 (6.02) |
40 (15.04) |
58 (21.80) |
80 (30.08) |
41 (15.41) |
5 (1.88) |
266 (100) |

Sex distribution
For the entire study period (1989~2018), 54 of the 266 patients (20.30%) were male and the remaining 212 patients (79.70%) were female, a ratio of 1:3.9. In terms of the subperiods, from 1989 to 1998, 17 of the 96 patients (17.71%) were male and 79 (82.29%) were female (1:4.6), while from 1999 to 2008, 17 of the 69 patients (24.64%) were male and 52 (75.36%) were female (1:3.1). Finally, from 2009 to 2018, 20 of the 101 patients (19.80%) were male and 81 (80.20%) were female (1:4.1).
Seasonal distribution
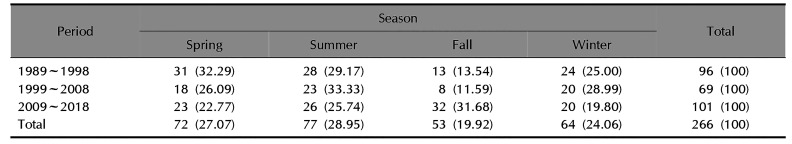
Table 2 shows that, from 1989 to 2018, 77 of the 266 patients (28.95%) visited hospital in summer, 72 (27.07%) in spring, 64 (24.06%) in winter, and 53 (19.92%) in fall. In the earlier subperiod (1989~1998), spring was the most common season for patients to visit hospital. From 1999 to 2008, summer was the most common season, and from 2009 to 2018, fall was the most common season.
Table 2
Seasonal distribution of adult patients with tinea capitis
|
Period |
Season |
Total |
|
Spring |
Summer |
Fall |
Winter |
|
1989~1998 |
31 (32.29) |
28 (29.17) |
13 (13.54) |
24 (25.00) |
96 (100) |
|
1999~2008 |
18 (26.09) |
23 (33.33) |
8 (11.59) |
20 (28.99) |
69 (100) |
|
2009~2018 |
23 (22.77) |
26 (25.74) |
32 (31.68) |
20 (19.80) |
101 (100) |
|
Total |
72 (27.07) |
77 (28.95) |
53 (19.92) |
64 (24.06) |
266 (100) |

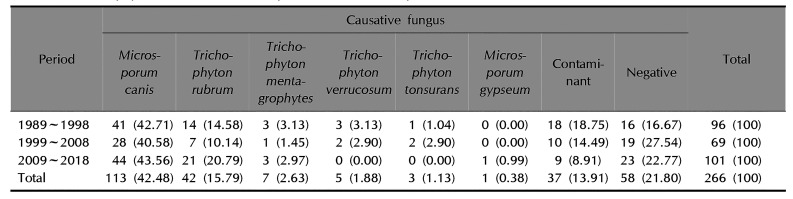
Isolation of dermatophytes
As can be seen in
Table 3, from 1989 to 2018,
M. canis was the most common dermatophyte (42.48%) isolated from tinea capitis patients, while
Trichophyton rubrum was the second most common (15.79%). From 1989 to 1998,
M. canis was the most common dermatophyte (42.71%), with
T. rubrum the second most common (14.58%). From 1999 to 2008,
M. canis was also the most common dermatophyte (40.58%), followed by
T. rubrum at 10.14%. From 2009 to 2018,
M. canis was the most common dermatophyte (43.56%) isolated from tinea capitis and
T. rubrum was the second most common (20.79%).
Table 3
Dermatophytes isolated from adult patients with tinea capitis
|
Period |
Causative fungus |
Total |
|
Microsporum canis |
Trichophyton rubrum |
Trichophyton mentagrophytes |
Trichophyton verrucosum |
Trichophyton tonsurans |
Microsporum gypseum |
Contaminant |
Negative |
|
1989~1998 |
41 (42.71) |
14 (14.58) |
3 (3.13) |
3 (3.13) |
1 (1.04) |
0 (0.00) |
18 (18.75) |
16 (16.67) |
96 (100) |
|
1999~2008 |
28 (40.58) |
7 (10.14) |
1 (1.45) |
2 (2.90) |
2 (2.90) |
0 (0.00) |
10 (14.49) |
19 (27.54) |
69 (100) |
|
2009~2018 |
44 (43.56) |
21 (20.79) |
3 (2.97) |
0 (0.00) |
0 (0.00) |
1 (0.99) |
9 (8.91) |
23 (22.77) |
101 (100) |
|
Total |
113 (42.48) |
42 (15.79) |
7 (2.63) |
5 (1.88) |
3 (1.13) |
1 (0.38) |
37 (13.91) |
58 (21.80) |
266 (100) |

Patient residence
Residence was divided based on Korean administrative district. For the entire study period, 186 of the 266 patients (69.92%) lived in urban areas and 80 (30.08%) lived in rural areas. From 1989 to 1998, 65 of the 96 patients (67.71%) lived in urban areas and 31 (32.29%) in rural areas. From 1999 to 2008, 56 of the 69 patients (81.16%) lived in urban areas and 13 (18.84%) lived in rural areas. From 2009 to 2018, 65 of the 101 patients (64.36%) lived in urban areas and 36 (35.64%) lived in rural areas.
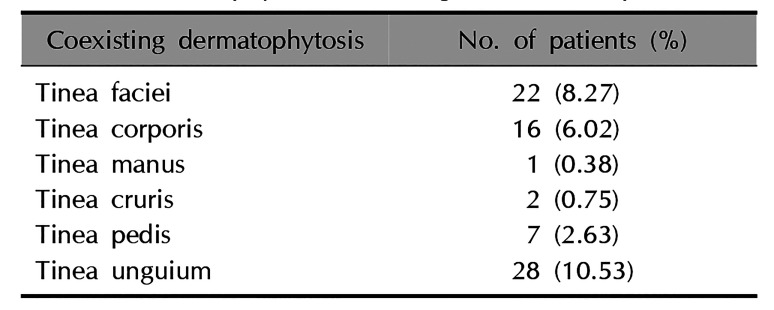
Coexisting dermatophytosis
A total of 101 patients from 2009 to 2018 were investigated for topographical distribution coexisting with tinea capitis (
Table 4). Tinea faciei coexisted with tinea capitis in 22 patients (8.27%) and tinea corporis coexisted in 16 patients (6.02%). Interestingly, tinea unguium coexisted in 28 patients (10.53%).
Table 4
Dermatophytosis coexisting with tinea capitis
|
Coexisting dermatophytosis |
No. of patients (%) |
|
Tinea faciei |
22 (8.27) |
|
Tinea corporis |
16 (6.02) |
|
Tinea manus |
1 (0.38) |
|
Tinea cruris |
2 (0.75) |
|
Tinea pedis |
7 (2.63) |
|
Tinea unguium |
28 (10.53) |

Clinical symptoms
A total of 101 patients from 2009 to 2018 presented with scaly hairless erythematous patches on the scalp. Ten patients exhibited kerion celsi, and 12 patients were misdiagnosed with seborrheic dermatitis.
Causative agents and underlying diseases
Of the 101 patients diagnosed with tinea capitis from 2009 to 2018, 9 had past contact history with cats. Some patients had underlying diseases; of these, 11 had diabetes mellitus, 28 had hypertension, and 13 had other underlying diseases.
Treatment of tinea capitis
From 2009 to 2018, all of the patients were treated with oral antifungal medicines such as terbinafine and itraconazole: 71 patients (70.30%) were treated with terbinafine, 25 (24.75%) with itraconazole, and 5 (4.95%) with both. All of them were treated 1~3 months, and then positive results in follow up KOH test prolonged the treatment duration. In the end, 60 patients were cured, 25 patients were lost during follow up period, and 2 patients were failed.
Comparison between patients infected with M. canis and T. rubrum
From 2009 to 2018, 41 patients were infected with M. canis and 21 patients with T. rubrum. M. canis infection was highest in their seventies (20 patients) and T. rubrum infection in their eighties (11 patients). M. canis infection was most common in spring (15 patients) and T. rubrum infection in winter (8 patients). M. canis infection developed kerion celsi but T. rubrum infection did not. Contact history of cats or other animals was noted only in M. canis infection. The ratio of cure to loss of follow up or failure during antifungal treatment was much higher in M. canis infection than in T. rubrum Infection.
Go to :

DISCUSSION
Our previous study reported that a total of 2,029 patients developed tinea capitis between 1978 and 1984. The number of adult patients with tinea capitis was only 41 (2.0%) in that study. However, the present study reveals that the annual incidence of tinea capitis in adults has increased over time from 41 (1978~1988) to 101 patients (2009~2018). In addition, the proportion of patients aged over 20 years from 1989 to 1998 was 12.31%, increasing to 25.75% from 1999 to 2008 and to 49.03% from 2009 to 2018. Compared with the proportion from 1978 to 1988, the proportion of adults with tinea capitis from 2009 to 2018 has thus increased tremendously. However, Cheon et al.
6 reported that the incidence of tinea capitis from 2016 to 2017 was still higher in children below 10 years old, while Kim et al.
7, Shin et al.
8, Chun et al.
9, and Kim et al.
10 also found that children below 10 years old were the most common patient group for tinea capitis. The hygiene of children has improved significantly compared to the past, so the incidence of tinea capitis has been lowered. However, the life patterns of adults have not changed and the life span increased. It explains increased incidence of adult patients with tinea capitis. In addition, the increasing incidence of adult tinea capitis may be attributed to the underlying diseases, such as diabetes, increased use of steroids and immunosuppressants, and the reduction of triglycerides with antifungal effects
4,
7. Our previous study found that 34 of the 41 adult tinea capitis patients were female. In this study, 54 of the 266 patients (20.30%) from 1989 to 2018 were male and the remaining 212 patients (79.70%) were female, while 20 of the 101 patients (19.80%) from 2009 to 2018 were male and 81 (80.20%) were female. Thus, there was a similar sex distribution for the disease across all of the subperiods. Kim et al.
7, Shin et al.
8, and Kim et al.
10 also reported that the incidence of tinea capitis was higher in adult females. In contrast, Chun et al.
9 found that the incidence of tinea capitis was higher in adult males. In addition, among children with tinea capitis, male patients are more common. In this study, female predominance in incidence of tinea capitis may be explained by hospital friendly tendency and prolonged life span. In addition, keeping pets and contact with them may be more frequent in female than in male.
Our previous study revealed that patients in their sixties had the highest incidence of tinea capitis
4. Similarly, in the present study, patients in their sixties were the most commonly inflicted age group from 1989 to 1998. However, from 2009 to 2018, the highest incidence rate was among patients in their seventies.
In a previous study, it was reported that patients with tinea capitis were diagnosed most commonly in winter
3. Kim et al.
7, Shin et al.
8, and Chun et al.
9 also reported that winter was the most common season for tinea capitis occurrence. However, patients with tinea capitis in the present study most commonly visited hospital in summer from 1989 to 2018, and from 2009 to 2018, patients with tinea capitis most commonly visited hospital in fall. Cutaneous fungal infections usually occur in a hot and humid weather. Therefore, incidence of the fungal infection may be highest in summer
11. However, incidence of tinea capitis can be influenced by changes in life style and accessibility to hospitals.
In our previous study,
M. canis (36 patients, 87.80%),
Trichophyton verrucosum (2 patients),
Trichophyton mentagrophytes (1 patient),
Trichophyton ferrugineum (1 patient), and
T. rubrum (1 patient) were found to be the causal agents for tinea capitis
4. In the present study,
M. canis was the most common dermatophyte (43.56%) isolated from tinea capitis from 2009 to 2018, followed by
T. rubrum (20.79%), both of which were also the two most common dermatophytes for the entire study period (42.48% and 15.79%, respectively). Cheon et al.
6 also reported that the second most common causative agent for tinea capitis was
T. rubrum. In addition, Kim et al.
7 and Shin et al.
8 reported the same results, though the proportion of
T. rubrum was lower in these studies compared to the present study. Chun et al.
9 reported that
T. mentagrophytes was the second most common causative agent for tinea capitis. Dermatophytes evolve along with socioeconomic conditions, population mobility and changes in human life style
12.
T. verrucosum is a frequent cause of dermatophytosis of cattle and other farm animals. Public health care and improved environmental conditions might lead to a decrease in the incidence of tinea capitis caused by
T. verrucosum.
Trichophyton tonsurans is a frequent cause of tinea capitis in teenagers, especially gladiators, such as judo players and wrestlers
13. Although tinea capitis caused by
T. tonsurans has spread to non-gladiators since 2000, it is still not common causative agent in adults.
T. rubrum is an anthropophilic dermatophyte and has been prevailing in Korea, constituting more than 90% of dermatophytoses since 1991
11.
In our previous study, tinea corporis (3 patients), tinea faciei (2 patients), and tinea pedis (1 patient) were found to coexist with tinea capitis
4. In the present study, from 2009 to 2018, tinea unguium (28 patients), tinea faciei (22 patients), tinea corporis (16 patients), tinea pedis (7 patients), tinea manus (1 patient), and tinea cruris (2 patients) coexisted with tinea capitis. These results differ from those reported by previous studies. For example, Kim et al.
7 and Shin et al.
8 reported that tinea faciei was the most common coexisting dermatophytosis.
Our previous study identified contact with cats (11 patients) and cows (2 patients) as causative factors
4. In the present study, of the 101 patients from 2009 to 2018, 9 had past contact history with cats. Some patients also had diabetes mellitus (11 patients), hypertension (28 patients), and other underlying diseases (13 patients). Kim et al.
7 and Shin et al.
8 reported that cats and dogs were the main causative factors for tinea capitis and that adult patients exhibited underlying diseases.
Griseofulvin was used as a therapeutic medication for tinea capitis before the introduction of new antifungal medicines such as terbinafine and itraconazole. Terbinafine is an effective agent in the treatment of tinea capitis because of its shorter treatment schedule and lower recurrence rate
14. Itraconazole is as effective as terbinafine in the treatment of tinea capitis
15,
16,
17, while ketoconazole should be avoided due to severe side effects
18. In the present study, the tinea capitis cases were treated effectively with oral terbinafine or itraconazole.
In summary, the epidemiological and mycological characteristics of adult patients with tinea capitis differ from those of children with tinea capitis in terms of annual incidence, sex distribution, and isolated dermatophytes. In particular, tinea capitis in adult patients showed increasing annual incidence, female predominance, and the increased proportion of T. rubrum among causative dermatophytes. This represents potentially useful information for the treatment and prevention of tinea capitis.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download