INTRODUCTION
ENDOCARDIAL APPROACH FOR BRUGADA SYNDROME
Characteristics of ventricular fibrillation-triggering premature ventricular complexes
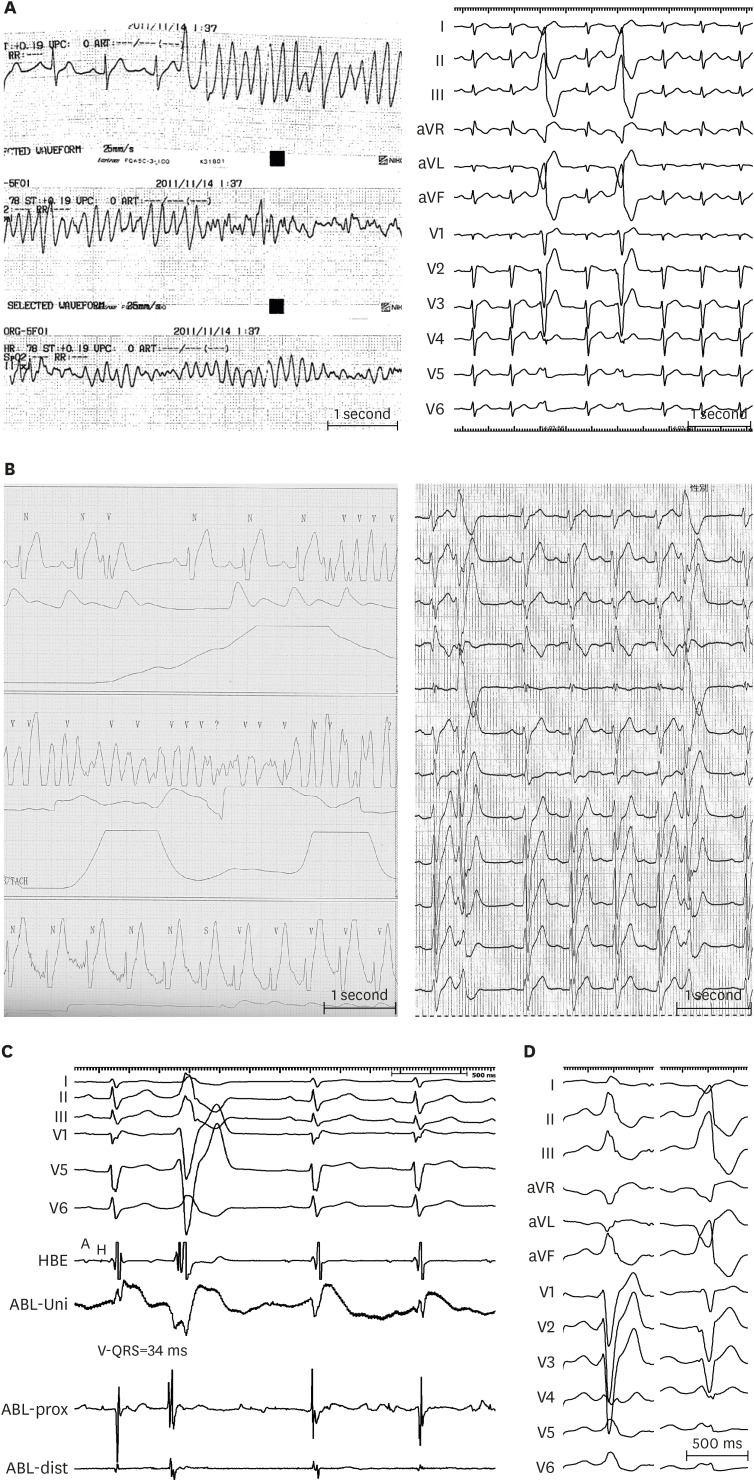 | Figure 1(A) Serial ECG strips of a BrS patient presented to the emergency room after resuscitation from VF. Frequent monofocal PVCs reproducibly degenerated into VF, leading to electrical storm. PVCs exhibited LBBB and inferior axis morphology. (B) Telemetry of a BrS patient presented with recurrent VF episodes. 12-lead ECG taken immediately after implantable cardioverter-defibrillator discharge showed PVCs with LBBB and superior axis configuration. (C) Intracardiac recordings at a successful ablation site of a VF-triggering PVC. The local ventricular activation recorded by the distal electrode pair of the ABL preceded the QRS complex onset by 34 ms with QS configuration of the unipolar electrogram. (D) VF-triggering PVC immediately after electrical storm (right) of a young male who underwent emergency VF-trigger ablation. Culprit PVC exhibits LBBB pattern and inferior axis. After culprit PVC elimination, VF became non-inducible after electrophysiology study with triple RV extra-stimuli. Pilsicainide infusion could induce a PVC of LBBB pattern, inferior axis indicating RV outflow tract origin (right); however the induced PVC morphology was different from VF-triggering PVC and was not ablated. The patient has been VF-free for 8-year follow-up.Modified from Talib AK, et al. Efficacy of endocardial ablation of drug-resistant ventricular fibrillation in Brugada syndrome: long-term outcome. Circ Arrhythm Electrophysiol 2018;11:e006675.15)
A = atrial potential; ABL = ablation catheter; aVF = augmented vector foot; aVL = augmented vector left; aVR = augmented vector right; BrS = Brugada syndrome; dist = distal bipole; ECG = electrocardiogram; H = his potential; HBE = his bundle electrogram; LBBB = left bundle-branch block; prox = proximal; PVC = premature ventricular complex; RV = right ventricular; Uni = unipolar electrogram; VF = ventricular fibrillation.
|
Ventricular fibrillation-trigger ablation in Brugada syndrome
Endocardial substrate ablation in Brugada syndrome
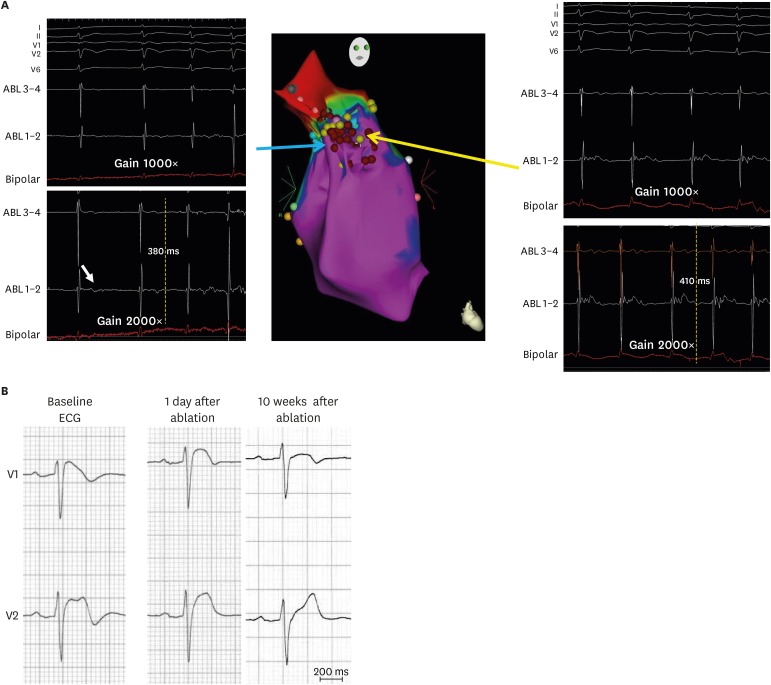 | Figure 2(A) Right anterior oblique view of 3-dimensional electroanatomical mapping of endocardial aspect of the RVOT where delayed low voltage fractionated EGMs were found at the RVOT lateral wall (red tags represent ablation points). Note these low voltage EGMs could be revealed using larger than the standard gain (2,000×) (B) Right precordial ECGs of a Brugada syndrome patient showing normalization of Brugada-type ST-segment elevation 10 weeks after endocardial ablation.Modified from Talib AK, et al. Efficacy of endocardial ablation of drug-resistant ventricular fibrillation in Brugada syndrome: long-term outcome. Circ Arrhythm Electrophysiol 2018;11:e006675.15)
ABL = ablation catheter; ECG = electrocardiogram; EGM = electrogram; RVOT = right ventricular outflow tract.
|
EPICARDIAL APPROACH
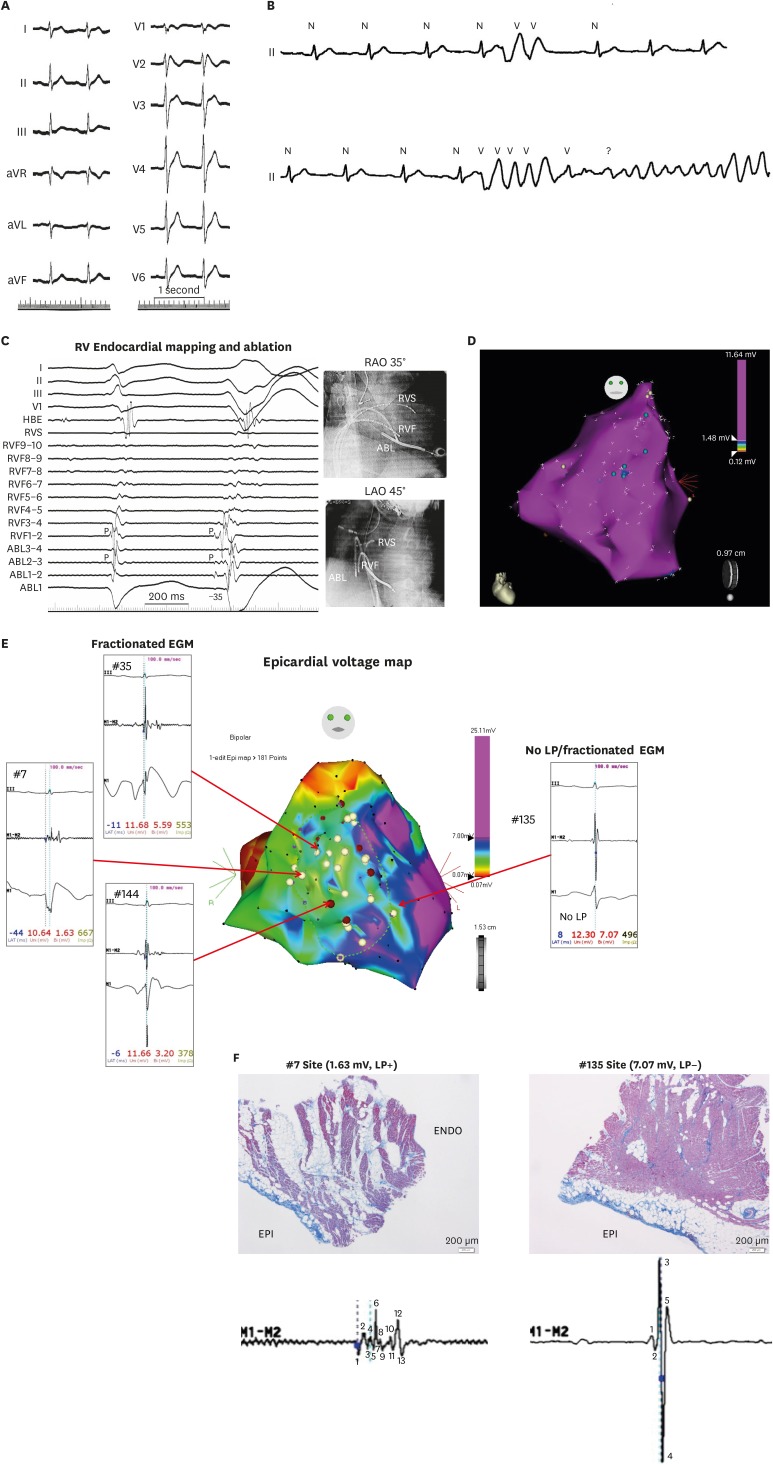 | Figure 3(A) A 12–lead electrocardiogram of a 27-year-old male with Brugada syndrome and SCN5A mutation Note QRS notching in lead V1. (B) After Pilsicainide infusion, Frequent PVCs were observed which degenerated into VF. (C) Mapping showed the earliest activation of the VF-triggering PVCs were observed at the RVF where presystolic Purkinje potential preceding the QRS onset of the PVC by 35 ms. Note that Purkinje potential is observed locally during sinus rhythm. (D) Endocardial electroanatomical mapping, repeated 8 years after the initial endocardial session, revealed normal RV/RV outflow tract voltage and confirmed elimination of previously ablated endocardial EGMs. Blue tags indicate remnant previously ablated LP. (E) Intraoperative epicardial mapping revealed relatively low voltage area with fractionated EGMs at the epicardial surface of previously ablated endocardial EGMs. Interestingly, coved-type ST-segment elevation was observed in the unipolar recordings of the ablation catheter at the fractionated EGM areas. (F) Epicardial biopsy and histology shows epicardial fibrosis with focal finger-like projections of collagen into myocardium while EGM-free areas revealed neither abnormal potential nor myocardial fibrosis.Modified from Talib AK, et al. Efficacy of endocardial ablation of drug-resistant ventricular fibrillation in Brugada syndrome: long-term outcome. Circ Arrhythm Electrophysiol 2018;11:e006675.15)
ABL = ablation catheter; aVF = augmented vector foot; aVL = augmented vector left; aVR = augmented vector right; EGM = electrogram; HBE = his bundle electrogram; LAO = left anterior oblique; LP = late potentials; PVC = premature ventricular complex; RAO = right anterior oblique; RVF = RV free wall; RV = right ventricular; RVS = RV septum; VF = ventricular fibrillation.
|
Epicardial substrate characteristics
Methods to enhance epicardial functional substrate
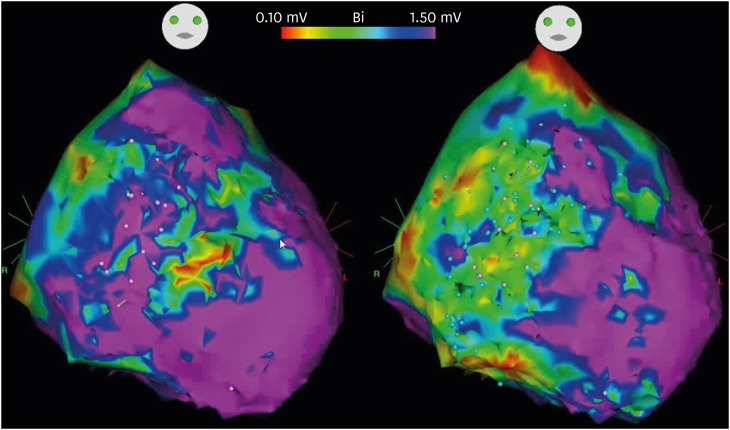 | Figure 4Electro-anatomical map of epicardial substrate of a 22-year-old male with Brugada syndrome. Baseline voltage map showed low voltage areas at the anterior and lateral aspects and RV and RVOT (left). After Pilsicainide infusion, the low voltage areas extended to involve almost the entire anterior and lateral aspects of RV and RVOT (right).RV = right ventricular; RVOT = right ventricular outflow tract.
|
Epicardial ablation endpoint
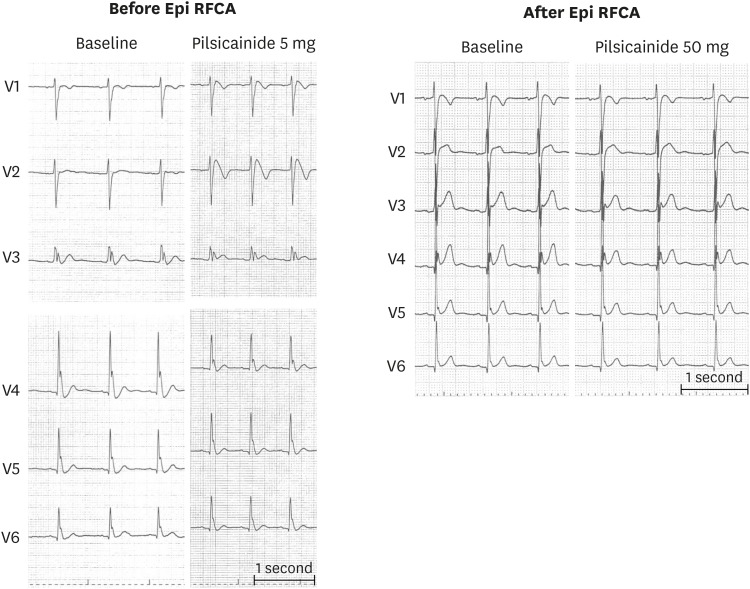 | Figure 5A 12-lead ECG of a young male who presented with electrical storm. Baseline ECG showed J-wave in leads V3–V6 and after very low dose (5 mg) Pilsicainide infusion, coved type ECG pattern was observed resulting in global type early repolarization (left). Despite extensive epicardial ablation at the RV/RV outflow tract with sodium channel blockade guide, Brugada ECG pattern could be eliminated even after higher dose Pilsicainide however, lateral chest lead J-wave could not be eliminated (right). Such patients may remain at high-risk of developing VF after ablation.ECG = electrocardiogram; RFCA = radiofrequency catheter ablation; RV = right ventricular; VF = ventricular fibrillation.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download