METHODS
Study population
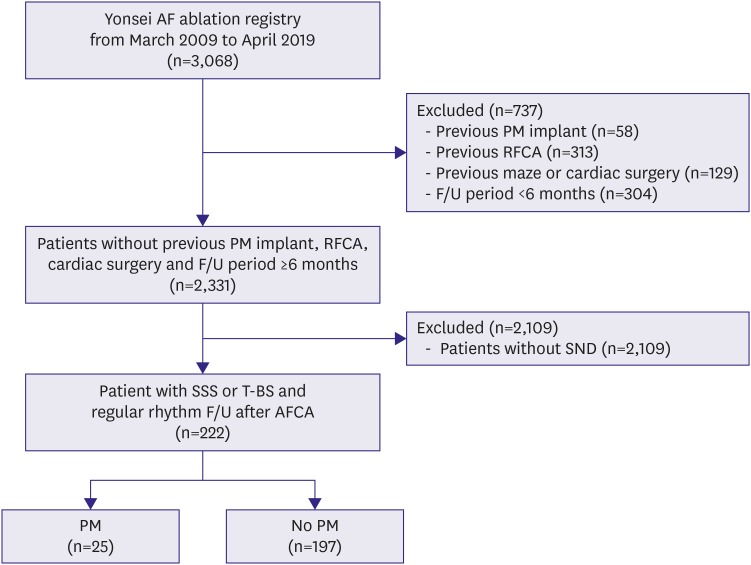
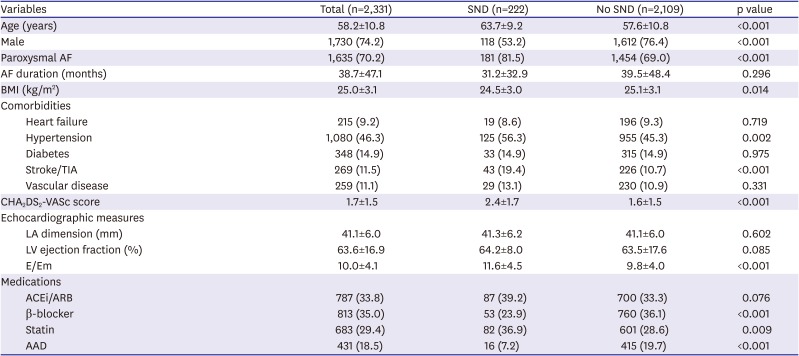
In this study, we defined sick sinus node syndrome or tachycardia-bradycardia syndrome as the inclusion criteria among a total of 3,068 patients out of the Yonsei AF ablation cohort who underwent catheter ablation due to AF from March 2009 to April 2019. After exclusion of 732 patients with 1) valvular AF, 2) previous permanent pacemaker implantation, 3) previous AFCA, 4) previous cardiac surgery or maze procedure, and 5) a post-AFCA follow-up duration of <6 months, 222 patients out of 2,331 with SND were included in this study (
Figure 1). SND was defined as symptomatic sinus bradycardia with a sinus rate under 50 beats per minute or sinus pauses of longer than 3 seconds with or without low dose antiarrhythmic drugs (AADs) to maintain sinus rhythm.
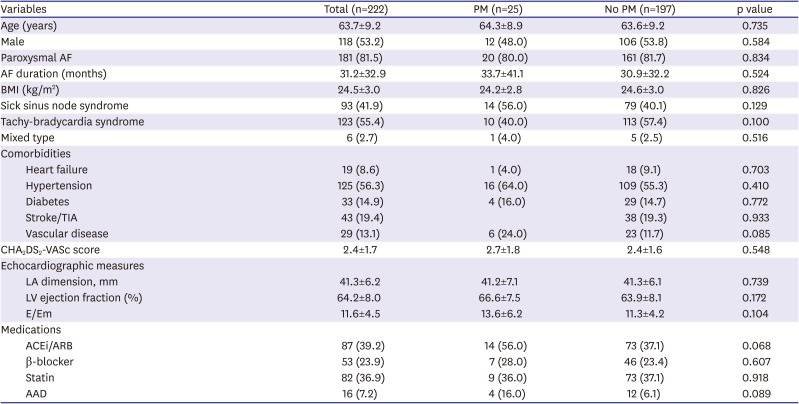
7) If the documented electrocardiogram (ECG) showed symptomatic SND immediately after AF termination, we classified it as tachycardia-bradycardia syndrome. We compared the patients who underwent a permanent pacemaker implantation with those who did not after the AFCA.
Figure 1
Flowchart of the patient analyses.
AFCA = atrial fibrillation catheter ablation; F/U = follow-up; PM = pacemaker; RFCA = radiofrequency catheter ablation; SND = sinus node dysfunction; SSS = sick sinus syndrome; TBS = tachycardia-bradycardia syndrome.
Electroanatomical mapping and radiofrequency catheter ablation
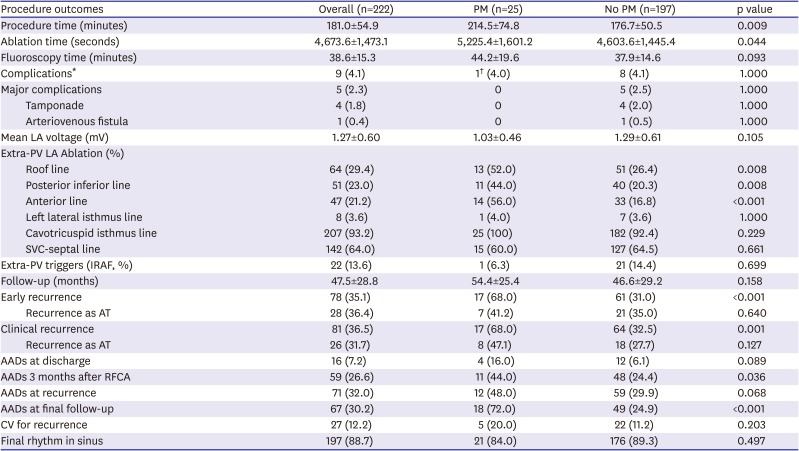
Three-dimensional (3D) electroanatomical mapping (NavX; Abbott Inc., Minnetonka, MN, USA) was generated using a circumferential pulmonary vein (PV)-mapping catheter (Lasso; Biosense Webster Inc., Diamond Bar, CA, USA) through a long sheath (Schwartz left 1; Abbott Inc.). The 3D geometry of both the left atrium and PVs was generated using the NavX system and then merged with 3D spiral computed tomography (CT) images. Blinded to the patient information, a technician analyzed the color-coded CT-merged NavX voltage maps using custom software (Image Pro; Media Cybernetics Inc., Rockville, MD, USA). An open-irrigated tip catheter (Celsius, Johnson & Johnson Inc., Diamond Bar, CA, USA; NaviStar ThermoCool, Biosense Webster Inc.; ThermoCool SF, Biosense Webster Inc.; ThermoCool SmartTouch, Biosense Webster Inc.; Coolflex, Abbott Inc.; 30–35 W; 47°C.; FlexAbility, Abbott Inc.; ThermoCool SmartTouch, Biosense Webster Inc.; and TactiCath, Abbott Inc.) was used for the AFCA. All patients underwent a de novo procedure with a circumferential PV isolation (CPVI). The majority of the patients underwent the creation of cavotricuspid isthmus (CTI) block during the de novo procedure unless there was AV conduction disease. As an extra-PV left atrial ablation, we conducted additional linear ablation including a roof line, posterior inferior line (posterior box lesion), and anterior line, particularly in patients with persistent AF. A left lateral isthmus ablation, right atrial ablation, and complex fractionated electrogram (CFAE) ablation were performed in the minority of the patients at the operator's discretion. We defined an extra-PV left atrial ablation as additional linear ablation with or without a CFAE ablation following the CPVI. The de novo procedure ended when there was no immediate recurrence of AF within 10 minutes after cardioversion with an isoproterenol infusion (5–10 μg/min).
Post-ablation management and follow-up
Patients visited the outpatient clinic at 1, 3, 6, and 12 months and every 6 months thereafter or whenever symptoms developed after the AFCA. ECG was performed at every visit. Twenty-four-hour Holter monitoring was performed at 3, 6, and 12 months and then every 6 months according to the 2012 Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society expert consensus statement guidelines.
8) Patients who suffered from symptoms of palpitations underwent Holter/event-monitor examinations to investigate the possibility of an arrhythmia recurrence. We defined an AF recurrence as any episode of atrial tachycardia or AF lasting for 30 seconds or more. Any electrocardiography documentation of an AF recurrence after the 3-month blanking period was classified as a clinical recurrence.
Statistical analysis
Continuous variables are expressed as the mean±standard deviation. Categorical variables are reported as the count (percentage). To compare the baseline characteristics and clinical outcomes between the 2 groups, we used the Student's t-test or Mann-Whitney test for continuous variables and the χ2 test for categorical variables. A Kaplan-Meier analysis with a log-rank test was used to compare the clinical recurrence rates according to the presence or absence of SND. The incidence of a pacemaker implantation and the rhythm outcomes after the catheter ablation were analyzed. All statistical analyses were performed using SPSS (Statistical Package for Social Sciences, Chicago, IL, USA) software for Windows (version 25.0).
DISCUSSION
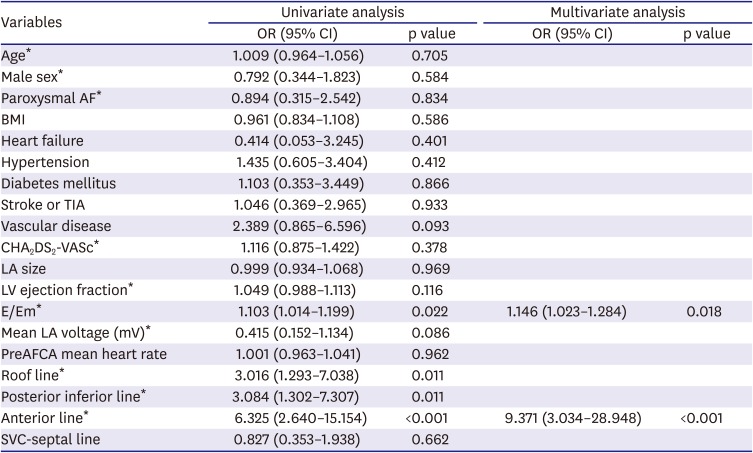
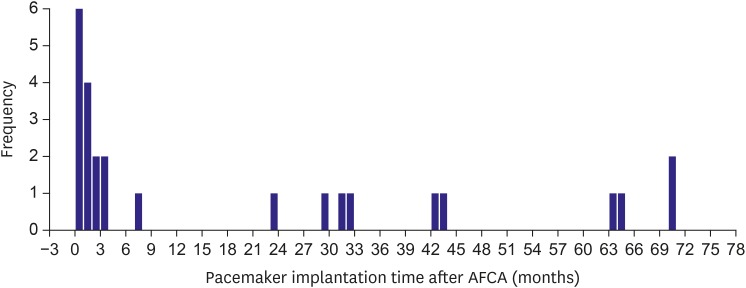
The main findings of this study were that 11.3% of the AF patients with SND eventually underwent a permanent pacemaker implantation after the AFCA during a mean follow-up period of 47.5±28.8-months, and 56% of them had permanent pacemakers within 3 months of the AFCA. The annual pacemaker implantation rate was 2.0% thereafter. Although the AF/AT recurrence rate after the AFCA between the patients with SND and those without exhibited no significant difference, the early and clinical recurrence rates and continuous AAD use were significantly higher in the permanent pacemaker implantation group than in the no pacemaker group among the patients with SND. We found that an anterior linear ablation during the de novo procedure and the E/Em were independently associated with a permanent pacemaker implantation after the AFCA in the patients with AF and SND.
Although SND is caused by functional or histological deterioration of the sinus node located in the right atrium, the primary mechanisms of AF triggers and the maintenance are known to be driven by the left atrium and PVs. Nevertheless, both AF and SND have much in common with each other. Recently,
PITX2, the top 1st common genetic loci of AF, has been implicated not only in the PV development
9) but has also been linked to the multiple genes (
TBX5,
Gja1, or
SCN5a) involved in the development of the sinus node and right and left atrial asymmetry.
10) Atrial arrhythmias associated with SND are known to be present in 40% to as much as 70% of those patients.
11) Many variables including structural and electrical remodeling, genetic mutations, a cholinergic effect, and reverse remodeling after AF rhythm control explain the SND accompanied by AF.
In patients with AF, remodeling and significant atrial fibrosis near the sinus node area are associated with clinically significant SND.
3) In previous studies, SND could be induced under conditions of pacing-induced chronic AF, a prolonged intra-atrial conduction time, and a decreased atrial refractoriness under sustained AF.
12) A prolonged sinus node recovery time and slower intrinsic heart rates were gradually reversed after termination of AF. Down-regulation of the sinoatrial node ion channel (
HCN, hyperpolarization-activated cyclic nucleotide-gated) caused by atrial tachycardia may result in a decreased expression of the ion channels in the sinus node, which operate as a pacemaker and may cause SND.
13)
Catheter ablation has been used to treat AF patients for several decades. Previous studies have shown that paroxysmal AF with SND can be treated by AFCA,
5) although the association and causality between AFCA in SND has not been fully elucidated. Sparks et al.
14) demonstrated that both paroxysmal and chronic atrial flutter exhibited an improvement in the sinus node recovery time 3 weeks after atrial flutter catheter ablation. Catheter ablation in patients with AF-induced bradycardia may reduce the need for AADs and a pacemaker implantation.
5) Thus, a successful reduction in the AF burden by AFCA leads to electrical reverse remodeling and reduces the permanent pacemaker requirement by reducing the use of drugs that suppress the SN function. However, SND associated with irreversible structural remodeling and replacement fibrosis may persist despite AF rhythm control,
15) eventually leading to a permanent pacemaker implantation. Although atrial scar burden might be related to the pacemaker requirement, LA voltage did not differ between pacemaker group and no pacemaker group with statistical significance and right atrial voltage data were not available in this study. Previously we reported post-AF ablation high sinus heart rate in patients with significant vagal modulation and its association with favorable rhythm outcome after catheter ablation.
16) In the present study, the mean heart rate was significantly increased in no pacemaker group, but not in the pacemaker group. There is one possibility that the increase in heart rate after AFCA ameliorated the pacemaker requirement in patients with significant vagal modulation.
However, since AFCA itself is a destructive surgery, iatrogenic aggravation of the SND due to atrial tissue damage or vascular injury to the sinus nodal artery cannot be excluded. A higher risk of post-operative SND after a bi-atrial maze procedure compared to a left atrial maze procedure was reported in patients who underwent mitral valve surgery.
17) In this study, the AFCA procedure time and ablation time were significantly longer in the pacemaker implantation group than in those without, and extra-PV ablation including an anterior line was independently associated with a permanent pacemaker implantation after the AFCA. Therefore, the cause and effect relationship between the higher AF recurrence rate and the eventual permanent pacemaker implantation group is not clear.
In a previous study that compared patients with SND and those without, the LA dimension in the SND patients was larger than that in those without SND.
18) Several studies have shown that factors including atrial remodeling and congestive heart failure that suggest stretched atria have an effect on the sinus node function.
19) In patients who underwent mitral valve surgery and concomitant maze procedures, post-operative SND that required a pacemaker was more commonly observed in patients with moderate to severe tricuspid regurgitation than in their counterparts.
17) This suggests that tricuspid regurgitation might contribute to right atrial and SN remodeling. In the present study, the E/Em, which indicates the diastolic function of the left ventricle, was independently associated with a permanent pacemaker implantation after the AFCA. We previously reported that a high E/Em and elevated LA pressure were significantly associated with atrial structural remodeling reflected by the LA volume and LA voltage and a higher recurrence of AF after the AFCA.
20)
The striking finding of this study was that an anterior linear ablation
21) was an independent risk factor for a permanent pacemaker implantation after the AFCA in patients with underlying SND. An anterior line is a linear lesion that connects the anterior mitral ring to the roof line. Pak et al.
21) reported that the bidirectional block rate and procedural success rate were significantly better after an anterior linear ablation than after a left lateral isthmus ablation in patients with persistent AF. We occasionally experienced post-ablation SND after the anterior linear ablation, but most SND recovered within 24 hours after the procedure. This may be due to the anterior line passing through the area of the sinus nodal artery, which exists parallel inside of Bachmann's bundle. However, we confirmed that the anterior line was the significant independent risk factor of a permanent pacemaker implantation after the AFCA in patients with AF and SND.
This study had several limitations. First, it is a retrospective study, conducted in a single center, and only included patients with concurrent AF and SND. Therefore, there could have been a selection bias. Second, elderly patients with severely symptomatic SND generally preferred AAD treatment after a pacemaker implantation rather than AFCA. Therefore, this study had a selection bias for AFCA, and the outcome of this study cannot be generalized to all patients with SND and AF. Third, although 24-hour Holter monitoring was performed at 3, 6, and 12 months and then every 6 months in both groups, the rhythm monitoring could have been more aggressive, and the detection sensitivity for AF recurrence was higher in the pacemaker group when the atrial high rate episodes were detected by the device than in no pacemaker group. Nonetheless, this study had implications for evaluating the long-term prognosis in patients with evidence-based indications for an AFCA prior to a pacemaker implantation.
After AFCA in patients with AF and SND, 1 out of 9 patients needed a pacemaker implantation and half underwent a placement within 3 months. The AF recurrence rate was significantly higher in the patients who required a pacemaker implantation after the AFCA. The causal-result relationship between the higher AF recurrence rate and the eventual permanent pacemaker implantation group is not clear.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download