1. Kartchner MM, McRae LP. Noninvasive evaluation and management of the “asymptomatic” carotid bruit. Surgery. 1977; 82:840–847. PMID:
929374.
2. Aboyans V, Ricco JB, Bartelink ME, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018; 39:763–816. PMID:
28886620.
3. Prevost TC, Abrams KR, Jones DR. Hierarchical models in generalized synthesis of evidence: an example based on studies of breast cancer screening. Stat Med. 2000; 19:3359–3376. PMID:
11122501.
4. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004; 23:3105–3124. PMID:
15449338.
5. Kougias P, Collins R, Pastorek N, et al. Comparison of domain-specific cognitive function after carotid endarterectomy and stenting. J Vasc Surg. 2015; 62:355–361. PMID:
26211378.
6. Rosenfield K, Matsumura JS, Chaturvedi S, et al. Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis. N Engl J Med. 2016; 374:1011–1020. PMID:
26886419.
7. Brott TG, Howard G, Roubin GS, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 2016; 374:1021–1031. PMID:
26890472.
8. Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008; 358:1572–1579. PMID:
18403765.
9. Marine LA, Rubin BG, Reddy R, Sanchez LA, Parodi JC, Sicard GA. Treatment of asymptomatic carotid artery disease: similar early outcomes after carotid stenting for high-risk patients and endarterectomy for standard-risk patients. J Vasc Surg. 2006; 43:953–958. PMID:
16678689.
10. Bradac O, Mohapl M, Kramar F, et al. Carotid endarterectomy and carotid artery stenting: changing paradigm during 10 years in a high-volume centre. Acta Neurochir (Wien). 2014; 156:1705–1712. PMID:
25011733.
11. Brewster LP, Beaulieu R, Corriere MA, et al. Carotid revascularization outcomes comparing distal filters, flow reversal, and endarterectomy. J Vasc Surg. 2011; 54:1000–1004. PMID:
21871772.
12. Tang GL, Matsumura JS, Morasch MD, et al. Carotid angioplasty and stenting vs carotid endarterectomy for treatment of asymptomatic disease: single-center experience. Arch Surg. 2008; 143:653–658. PMID:
18645107.
13. Yang SS, Kim YW, Kim DI, et al. Impact of contralateral carotid or vertebral artery occlusion in patients undergoing carotid endarterectomy or carotid artery stenting. J Vasc Surg. 2014; 59:749–755. PMID:
24360588.
14. Zarins CK, White RA, Diethrich EB, et al. Carotid revascularization using endarterectomy or stenting systems (CaRESS): 4-year outcomes. J Endovasc Ther. 2009; 16:397–409. PMID:
19702339.
15. Kim LK, Yang DC, Swaminathan RV, et al. Comparison of trends and outcomes of carotid artery stenting and endarterectomy in the United States, 2001 to 2010. Circ Cardiovasc Interv. 2014; 7:692–700. PMID:
25116802.
16. Bisdas T, Egorova N, Moskowitz AJ, et al. The impact of gender on in-hospital outcomes after carotid endarterectomy or stenting. Eur J Vasc Endovasc Surg. 2012; 44:244–250. PMID:
22819738.
17. Yuo TH, Degenholtz HS, Chaer RA, Kraemer KL, Makaroun MS. Effect of hospital-level variation in the use of carotid artery stenting versus carotid endarterectomy on perioperative stroke and death in asymptomatic patients. J Vasc Surg. 2013; 57:627–634. PMID:
23312937.
18. Vouyouka AG, Egorova NN, Sosunov EA, et al. Analysis of Florida and New York state hospital discharges suggests that carotid stenting in symptomatic women is associated with significant increase in mortality and perioperative morbidity compared with carotid endarterectomy. J Vasc Surg. 2012; 56:334–342. PMID:
22583852.
19. McDonald RJ, McDonald JS, Therneau TM, Lanzino G, Kallmes DF, Cloft HJ. Comparative effectiveness of carotid revascularization therapies: evidence from a National Hospital Discharge Database. Stroke. 2014; 45:3311–3319. PMID:
25300973.
20. Jim J, Rubin BG, Ricotta JJ 2nd, et al. Society for Vascular Surgery (SVS) Vascular Registry evaluation of comparative effectiveness of carotid revascularization procedures stratified by Medicare age. J Vasc Surg. 2012; 55:1313–1320. PMID:
22459755.
21. Choi JC, Johnston SC, Kim AS. Early outcomes after carotid artery stenting compared with endarterectomy for asymptomatic carotid stenosis. Stroke. 2015; 46:120–125. PMID:
25424479.
22. Nolan BW, De Martino RR, Goodney PP, et al. Comparison of carotid endarterectomy and stenting in real world practice using a regional quality improvement registry. J Vasc Surg. 2012; 56:990–996. PMID:
22579135.
23. Walker MD, Marler JR, Goldstein M, et al. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995; 273:1421–1428. PMID:
7723155.
24. Hobson RW 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. N Engl J Med. 1993; 328:221–227. PMID:
8418401.
25. Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010; 376:1074–1084. PMID:
20870099.
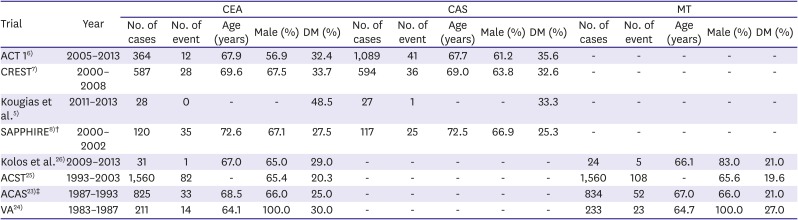
26. Kolos I, Troitskiy A, Balakhonova T, et al. Modern medical treatment with or without carotid endarterectomy for severe asymptomatic carotid atherosclerosis. J Vasc Surg. 2015; 62:914–922. PMID:
26410046.
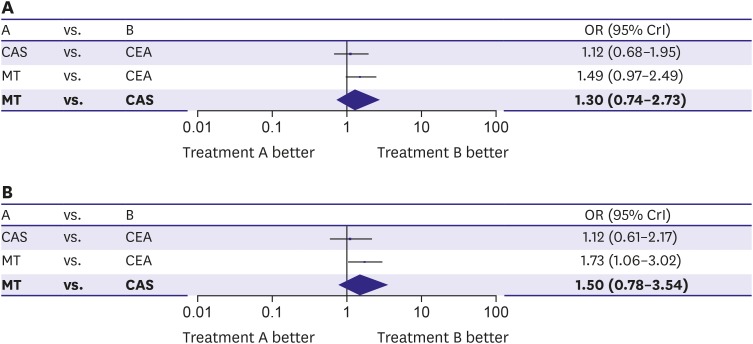
27. Raman G, Moorthy D, Hadar N, et al. Management strategies for asymptomatic carotid stenosis: a systematic review and meta-analysis. Ann Intern Med. 2013; 158:676–685. PMID:
23648949.
28. Sardar P, Chatterjee S, Aronow HD, et al. Carotid artery stenting versus endarterectomy for stroke prevention: a meta-analysis of clinical trials. J Am Coll Cardiol. 2017; 69:2266–2275. PMID:
28473130.
29. Song F, Xiong T, Parekh-Bhurke S, et al. Inconsistency between direct and indirect comparisons of competing interventions: meta-epidemiological study. BMJ. 2011; 343:d4909. PMID:
21846695.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download