INTRODUCTION
MAIN INDICATIONS TO PULMONARY VALVE INTERVENTIONS
HISTORICAL OVERVIEW
PREOPERATIVE EVALUATION
• Surface electrocardiogram (EKG) and 24-h Holter EKG monitoring to detect arrhythmia and define QRS duration.
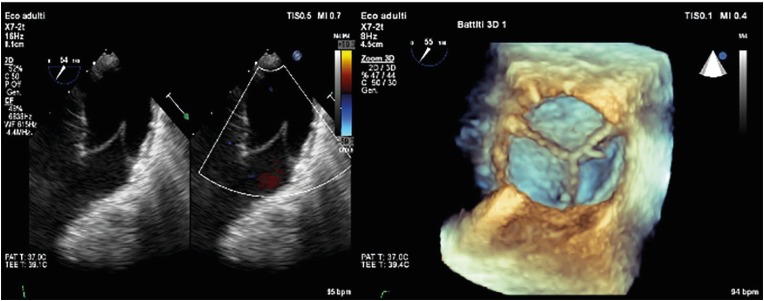
• Echocardiography as the first screening tool. On one hand, it allows to determine the presence of a residual RVOT stenosis identifying the site, quantifying the severity and determining the cause of the stenosis itself. The estimation of the systolic pressure gradient is derived from the trans pulmonary velocity flow curve using the simplified Bernoulli equation (DP=4v2)), applying continuous-wave Doppler parallel to the flow across the RVOT usually through a parasternal short axis view or, less frequently, through a subcostal window. The grade of stenosis is defined as follow: severe with a peak jet velocity >4 m/s (peak gradient>64 mmHg), moderate with a peak jet velocity of 3–4 m/s (peak gradient 36–64 mmHg), mild with a peak jet velocity <3 m/s (peak gradient less than 36 mmHg).14) On the other hand, echocardiography allows to assess the severity of PR according to the regurgitant jet width (if more than 65% of the RVOT, is in favour of severe PR) and to the deceleration of continuous wave signal of PR jet assessed qualitatively (steep deceleration is in favour of severe PR).15) Moreover, this non-invasive tool is also used to evaluate RV dimensions (measuring RV diameters at the base and at the mid-level and the long axis in a 4-chamber view and quantifying the RVOT diameter in a parasternal short axis view), RV systolic function (usually through tricuspid annular plane systolic excursion, S′ wave peak at the tissue Doppler imaging or fractional area change) and to estimate the RV pressure from tricuspid valve regurgitant jet (TR velocity>2.8–2.9 m/s, assuming an RA pressure of 3–5 mmHg, indicates elevated RV systolic pressure) and the RV to systemic pressure ratio. Echocardiography allows also the assessment of the left chambers end of associated lesions.16)
• Cardiopulmonary exercise testing on a bicycle using a ramp protocol. It provides a comprehensive assessment of the exercise response, and reflects the influences and interactions of the cardiac, respiratory, musculoskeletal and haematological systems. This test provides data on respiratory gas exchange, including oxygen uptake (VO2), carbon dioxide output (VCO2), tidal volume, minute ventilation (VE), oxygen pulse, ventilatory anaerobic threshold, and respiratory quotient and other variables such as EKG trace, blood pressure and oxygen saturation.17)18)
• Cardiovascular magnetic resonance imaging (MRI), providing that it is not contraindicated, is the gold standard to quantify PR and right ventricular volumes and function. Because of its complex anatomy, the RV is studied best in cardiac long-axis as well as in short axis direction. End-diastolic and end-systolic volumes and derived indices such as stroke volume, ejection fraction and myocardial mass are obtained by contouring endocardial and epicardial contours. Regional right ventricular function is described in terms of radial and longitudinal wall motion patterns, which can be assessed either visually or using a centreline method. Volumetric and functional right ventricular assessment is usually part of a more comprehensive right ventricular assessment.19)
• Computed tomography (CT) scan is performed in complex anatomy to evaluate the relationship between the pulmonary trunk and the aortic root, to assess coronary anatomy and RVOT size. Moreover, it is possible to estimate the risk of coronary artery compression according to the distance between the coronary artery and the landing zone.20)
• Three-dimensional (3D) printing. Candidates to PPVI can have complex post-surgical anatomies, therefore further imaging approaches can be necessary in order to have as many information as possible prior the percutaneous intervention. In addition to the standard imaging techniques previously reported, 3D printing is a useful tool that can help planning the PPVI. 3D printing (also referred to as rapid prototyping, stereolithography, or additive manufacturing) is a technology which fabricates a physical model from a 3D computerised imaging source file, usually MRI, CT or echocardiographic examination. Fusion of different modalities (e.g. ventricles from CT, valves from echocardiography) to create a single 3D model has been reported. The subsequent post processing image reworking is of fundamental importance. It consists of image segmentation and reconstruction in order to obtain a 3D patient-specific digital model of the target anatomic structures. Then, a 3D model can be printed.21)22) Its application for the study of the RVOT and the simulation of PPVI preceded by a pre-stenting intervention, to better understand the anatomy and the complex relationship with the coronary arterial course, has already been reported.23)
AVAILABLE PROSTHESIS
Figure 1
Melody valve. A modified bovine giugular vein with valve segment sutured on Numed Platinum Iridium stent; the stent can be crimped down to 6 mm, mounted on a BIB balloon and re-expanded up to 18, 20, and 22 mm. The delivery system is shown in the lower part of the figure.
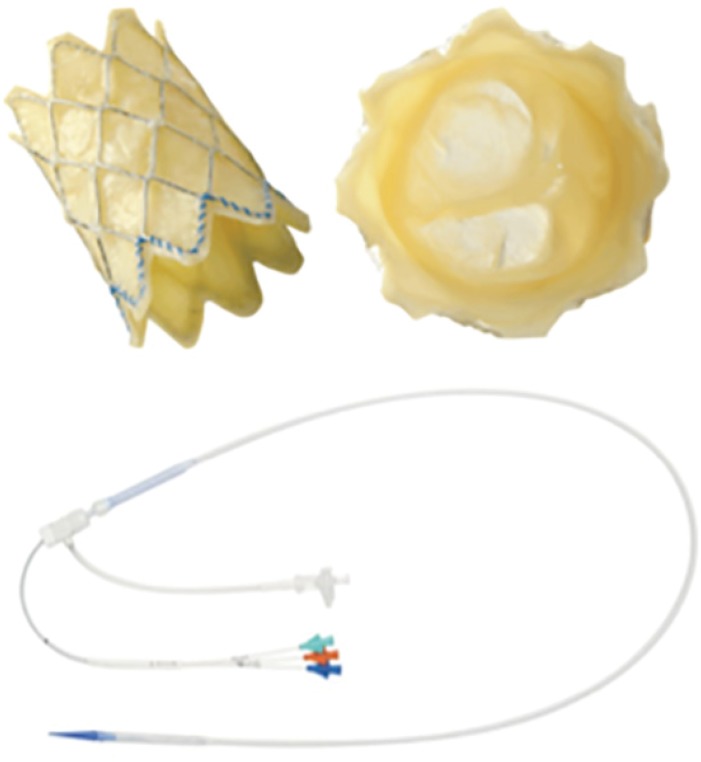
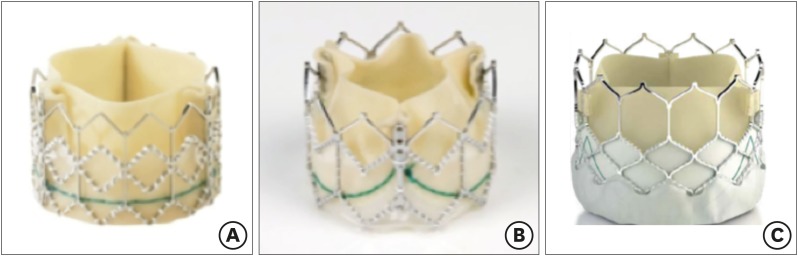
Figure 2
The evolution of Edwards Sapien Valve. (A) Edwards Sapien, (B) Edwards Sapien XT, and (C) Edwards Sapien 3.
THREE-DIMENSIONAL ROTATIONAL ANGIOGRAPHY
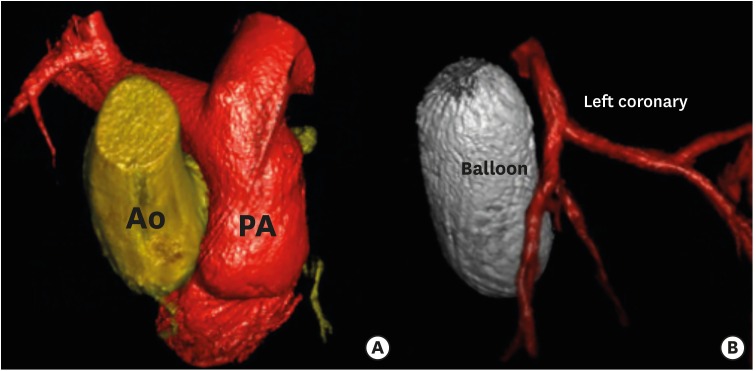
Figure 3
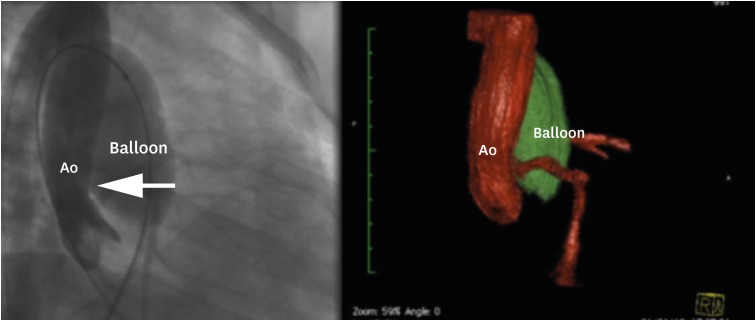
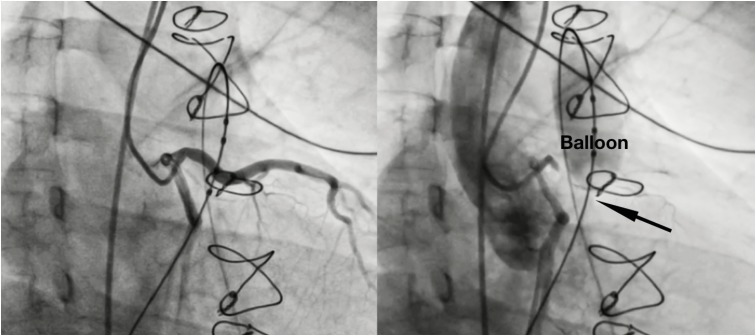
3D rotational angiography and 3D Examples of reconstruction. (A) Ao and PA reconstruction. (B) Relationship between a balloon inflated into the right ventricular outflow tract (ballon) and the left anterior descending artery (left coronary).
TECHNIQUE
Figure 6
Prestenting + Melody. (A) Right ventricular angiogram in a case of stenotic right ventricular to pulmonary artery conduit (arrow). (B) Angiogram after Melody implantation (black arrow), after prestenting with bare metal stent Andra (white arrow).
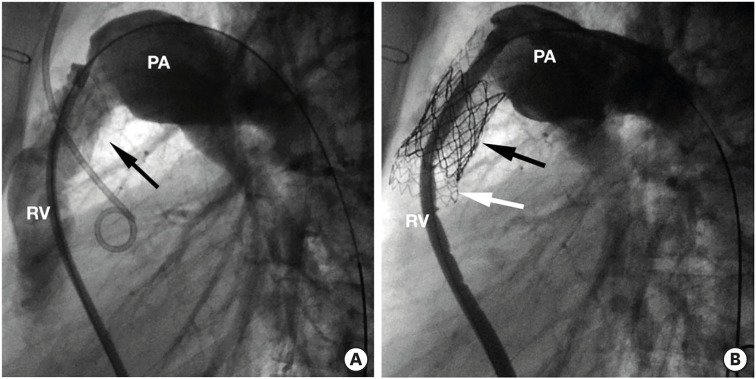
Figure 7
Melody implantation + prestenting with covered stent in a conduit with extensive calcifications (arrows). (A) Basal angiogram. (B) Angiogram after Melody implantation.
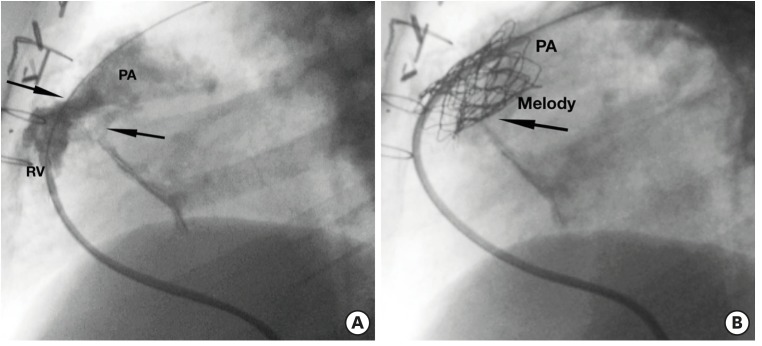
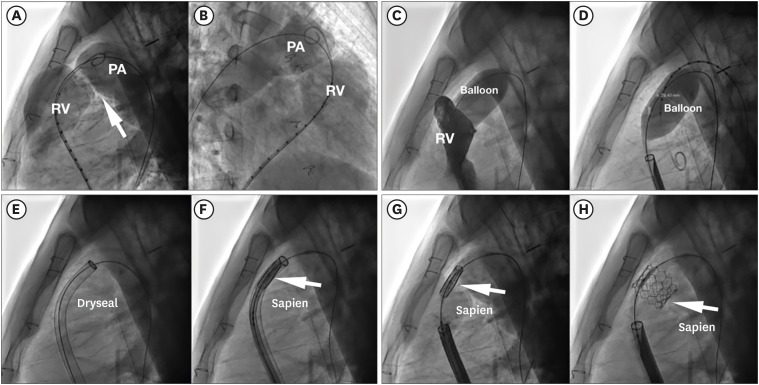
Figure 8
Step by step Sapien valve implantation tecnique in native RVOT. (A, B) Basal RVOT angiography. (C, D) Balloon inflation test + simultaneous right ventricular injection, showing no residual dye passage. (E) Dryseal long sheath advanced up to main pulmonary artery. (F) Sapien valve advanced into the sheath (arrow). (G, H) Sapien valve implanted (arrow).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download