INTRODUCTION
The effect of implantable cardioverter-defibrillator (ICD) therapy for improving survival in patients who have resuscitated sudden cardiac death (SCD) is well proven.
123 ICD therapy is also beneficial in high risk patients with ischemic or non-ischemic cardiomyopathy (CMP) with severe left ventricular dysfunction.
456 Current guidelines recommend ICD therapy for primary prevention of SCD in patients with severe left ventricular dysfunction with heart failure despite guideline-directed management and therapy,
7 and following these current guideline recommendations, medical insurance coverage by the Korean government for ICD therapy for primary prevention has been approved from 2008. Two previous Korean population studies showed the efficacy of ICD therapy for primary prevention, however these studies were performed in single centers with a small number patients.
89 As previously published, our multicenter study showed effectiveness of ICD therapy for heart failure patients with ischemic or non-ischemic CMP in Korea, however the number of ICD for primary prevention was also too small.
10 Therefore this study was done to evaluate the efficacy of ICD therapy for primary prevention compared to those for secondary prevention and to stratify patients who are benefiting from ICD therapy for primary prevention.
METHODS
Study design
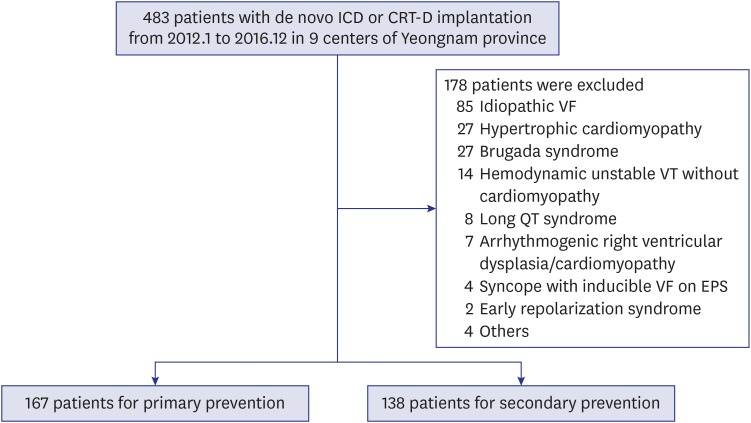
Between January 2012 and December 2016, 483 consecutive patients who received new implantation of ICD or cardiac resynchronization therapy-defibrillator (CRT-D) enrolled in a multicenter registry performed in 9 centers of Yeoungnam province, which is located in southeast of Korea (Andong General Hospital, Daegu Catholic University Medical Center, Daegu Fatima General Hospital, Dong-A University Hospital, Inje University Busan Haeundae Paik Hospital, Keimyung University Dongsan Medical Center, Kyungpook National University Hospital, Pusan National University Hospital, Yeungnam University Medical Center), were retrospectively analyzed. We excluded 178 patients with the following etiologies: idiopathic ventricular fibrillation (VF) (n = 85), hypertrophic CMP (n = 27), Brugada syndrome (n = 27), hemodynamic unstable ventricular tachycardia (VT) (n = 14), long QT syndrome (n = 8), arrhythmogenic right ventricular dysplasia/CMP (n = 7), syncope with inducible VF (n = 4), early repolarization syndrome (n = 2), and others (n = 4). Finally, 305 enrolled patients were divided into two groups according to indication of ICD implantation: primary prevention (n = 167) versus secondary prevention (n = 138) (
Fig. 1).
Fig. 1
Study flow chart. Patients with reduced left ventricular systolic function and/or documented VF/VT were enrolled and divided into primary prevention and secondary prevention groups.
VF = ventricular fibrillation, VT = ventricular tachycardia, ICD = implantable cardioverter-defibrillator, CRT-D = cardiac resynchronization therapy-defibrillator, EPS = electrophysiology studies.
Primary prevention was defined as ICD implantation in patients with left ventricular ejection fraction (LVEF) less than 30%, or 35% with heart failure symptom of New York Heart Association (NYHA) functional class II, III. Secondary prevention was defined as ICD implantation in patients who were resuscitated SCD due to documented VF/VT and had ischemic or non-ischemic CMP. Ischemic CMP was defined as presence of previous coronary artery disease including myocardial infarction with or without revascularization. Non-ischemic CMP was defined as presence of LVEF less than 50% without significant coronary artery disease in secondary prevention. Demographic and clinical characteristics including age, gender, body mass index (BMI), and NYHA functional class, cardiovascular risk factors and comorbidities (hypertension, diabetes, previous ischemic heart disease, and chronic obstructive pulmonary disease), and laboratory test, echocardiographic and electrocardiographic findings were identified. Ischemic CMP was defined as presence of previous myocardial infarction or significant coronary artery stenosis on angiography. Type of ICD such as single chamber, dual chamber, and CRT-D was also evaluated.
Outcomes
We assessed appropriate ICD therapy rate including shock and anti-tachycardia pacing (ATP), inappropriate shock rate, and all-cause deaths which were the sum of cardiac and non-cardiac death. Also, we investigated the predictor for death before appropriate ICD shock in the primary prevention group. Follow-up data were obtained by reviewing the medical records during routine outpatient visits at each center.
Statistical analysis
Data are presented as mean ± standard deviation for continuous variables and percentages for categorical variables. All comparisons between baseline variables were made using Student's t-test for continuous variables and the χ2 test for categorical variables. Event free survivals including appropriate ICD therapy, inappropriate ICD shock, and all-cause death were analyzed by the Kaplan-Meier method. The P values were 2-sided, and P < 0.05 was considered significant. A multivariate Cox-proportional hazard model was used to identify independent predictors for all-cause death. Patients were categorized as with and without death before appropriate ICD shock. Receiver-operating characteristics (ROC) curve analysis was used for determination of the cut-off values for prediction of death before appropriate ICD shock. Statistical analysis was performed using SPSS version 20.0 for Windows (SPSS Inc., Chicago, IL, USA).
Ethics statement
The present study protocol was reviewed and approved by the each Institutional Review Board including Kyungpook National University Hospital (approval No. KNUH 2017-06-002). Informed consent was waived by the board.
RESULTS
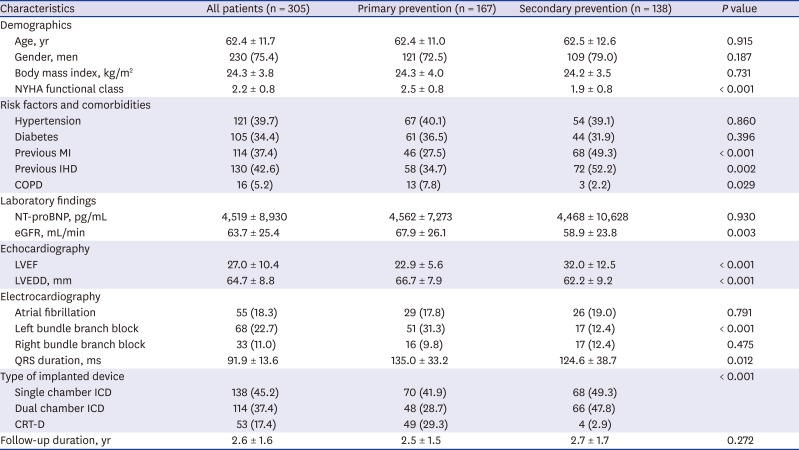
The baseline patient characteristics are shown in
Table 1. The mean age was 64 ± 12 years and 230 (75%) patients were men. Among secondary prevention patients, 52 and 83 experienced VF and sustained VT, respectively, before ICD implantation. The mean NYHA functional class and LVEF were 2.2% ± 0.8% and 27% ± 10%, respectively. Single chamber, dual chamber ICD, and CRT-D were implanted in 45%, 37%, and 17% of patients, respectively. Among overall patients, the etiology of 130 (42.6%) patients was ischemic and the mean follow-up duration was 2.6 ± 1.6 years. Compared with the secondary prevention group, NYHA function class was significantly higher (2.5 ± 0.8 vs. 1.9 ± 0.8,
P < 0.001) and the frequency of ischemic etiology was lower (34.7% vs. 52.2%,
P = 0.002) in the primary prevention group. Left bundle branch block on electrocardiography was more frequent (31.3% vs. 12.4%,
P < 0.001), and LVEF was lower (23% ± 5.6% vs. 32% ± 13%,
P < 0.001) and left ventricular end diastolic dimension (LVEDD) was larger (67 ± 7.9 mm vs. 62 ± 9.2 mm,
P < 0.001) in primary prevention group. CRT-D was more frequently implanted in the primary prevention group (29.3% vs. 2.9%,
P < 0.001). However, no significant differences were found between the groups regarding age, gender, BMI, histories of hypertension and diabetes, level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), and frequencies of atrial fibrillation and right bundle branch block. Also, there was no difference in follow-up duration between the groups.
Table 1
Patient characteristics
|
Characteristics |
All patients (n = 305) |
Primary prevention (n = 167) |
Secondary prevention (n = 138) |
P value |
|
Demographics |
|
|
|
|
|
Age, yr |
62.4 ± 11.7 |
62.4 ± 11.0 |
62.5 ± 12.6 |
0.915 |
|
Gender, men |
230 (75.4) |
121 (72.5) |
109 (79.0) |
0.187 |
|
Body mass index, kg/m2
|
24.3 ± 3.8 |
24.3 ± 4.0 |
24.2 ± 3.5 |
0.731 |
|
NYHA functional class |
2.2 ± 0.8 |
2.5 ± 0.8 |
1.9 ± 0.8 |
< 0.001 |
|
Risk factors and comorbidities |
|
|
|
|
|
Hypertension |
121 (39.7) |
67 (40.1) |
54 (39.1) |
0.860 |
|
Diabetes |
105 (34.4) |
61 (36.5) |
44 (31.9) |
0.396 |
|
Previous MI |
114 (37.4) |
46 (27.5) |
68 (49.3) |
< 0.001 |
|
Previous IHD |
130 (42.6) |
58 (34.7) |
72 (52.2) |
0.002 |
|
COPD |
16 (5.2) |
13 (7.8) |
3 (2.2) |
0.029 |
|
Laboratory findings |
|
|
|
|
|
NT-proBNP, pg/mL |
4,519 ± 8,930 |
4,562 ± 7,273 |
4,468 ± 10,628 |
0.930 |
|
eGFR, mL/min |
63.7 ± 25.4 |
67.9 ± 26.1 |
58.9 ± 23.8 |
0.003 |
|
Echocardiography |
|
|
|
|
|
LVEF |
27.0 ± 10.4 |
22.9 ± 5.6 |
32.0 ± 12.5 |
< 0.001 |
|
LVEDD, mm |
64.7 ± 8.8 |
66.7 ± 7.9 |
62.2 ± 9.2 |
< 0.001 |
|
Electrocardiography |
|
|
|
|
|
Atrial fibrillation |
55 (18.3) |
29 (17.8) |
26 (19.0) |
0.791 |
|
Left bundle branch block |
68 (22.7) |
51 (31.3) |
17 (12.4) |
< 0.001 |
|
Right bundle branch block |
33 (11.0) |
16 (9.8) |
17 (12.4) |
0.475 |
|
QRS duration, ms |
91.9 ± 13.6 |
135.0 ± 33.2 |
124.6 ± 38.7 |
0.012 |
|
Type of implanted device |
|
|
|
< 0.001 |
|
Single chamber ICD |
138 (45.2) |
70 (41.9) |
68 (49.3) |
|
Dual chamber ICD |
114 (37.4) |
48 (28.7) |
66 (47.8) |
|
CRT-D |
53 (17.4) |
49 (29.3) |
4 (2.9) |
|
Follow-up duration, yr |
2.6 ± 1.6 |
2.5 ± 1.5 |
2.7 ± 1.7 |
0.272 |
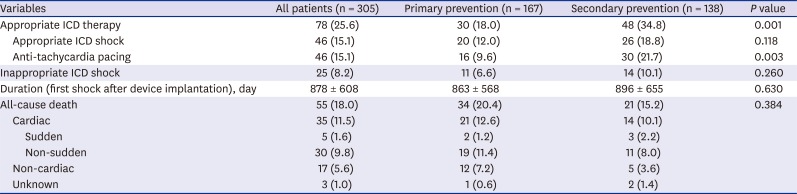
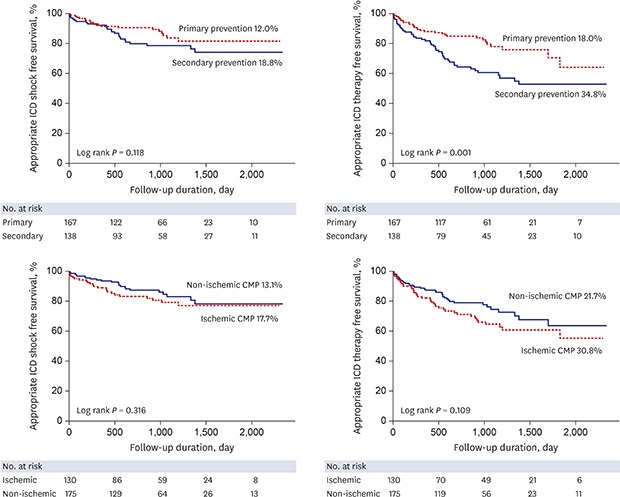
During mean follow-up duration of 2.6 ± 1.6 years, appropriate ICD therapy occurred in 78 patients (25.6%), and appropriate ICD shock and ATP occurred in 15.1% and 15.1% of patients, respectively (
Table 2). Appropriate ICD shock rate was not different between the groups (primary prevention 12% vs. secondary prevention 18.8%,
P = 0.118) (
Fig. 2). However, ATP rate was significantly higher in the secondary prevention group (primary prevention 9.6% vs. secondary prevention 21.7%,
P = 0.003). Among 83 secondary prevention patients who experienced sustained VT before ICD implantation, 19 (22.9%) experienced ATP without appropriate ICD shock. Appropriate ICD therapy rate including shock and ATP was significantly higher (primary prevention 18% vs. secondary prevention 34.8%,
P = 0.001) in the secondary prevention group. When analyzed according to the etiology, there was no difference in appropriate ICD shock and therapy between ischemic and non-ischemic etiology. Also, there was no difference in appropriate ICD shock rate according to ischemic or non-ischemic etiology in both the primary and secondary prevention groups (
Supplementary Fig. 1). There was no difference in inappropriate ICD shock rate between the primary and secondary prevention groups (6.6% vs. 10.1%,
P = 0.260). All-cause death occurred in 55 patients (18%) during follow-up periods. There was no difference in all-cause death rate (20.4% vs. 15.2%,
P = 0.384) including cardiac and non-cardiac death between the primary prevention and secondary prevention groups. Also appropriate ICD shock/therapy, inappropriate ICD shock, and ischemic etiology did not affect all-cause death (
Fig. 3 and
Supplementary Fig. 2). In the primary prevention group, there were no significant differences in all-cause death, appropriate and inappropriate ICD therapy according to the presence or absence of CRT-D. In univariate analysis, age, BMI, NYHA functional class, diabetes, estimated glomerular filtration rate (eGFR), log NT-proBNP, LVEF, and LVEDD was associated with all-cause death (
Table 3). In the Cox-proportional hazard model, BMI (hazard ratio [HR], 0.903; 95% confidence interval [CI], 0.820–0.996;
P = 0.041), diabetes (HR, 2.313; 95% CI, 1.298–4.121;
P = 0.004), and eGFR (HR, 0.976; 95% CI, 0.960–0.992;
P = 0.004), were independent predictors for all-cause death.
Table 2
Study outcomes according to the indication of ICD implantation
|
Variables |
All patients (n = 305) |
Primary prevention (n = 167) |
Secondary prevention (n = 138) |
P value |
|
Appropriate ICD therapy |
78 (25.6) |
30 (18.0) |
48 (34.8) |
0.001 |
|
Appropriate ICD shock |
46 (15.1) |
20 (12.0) |
26 (18.8) |
0.118 |
|
Anti-tachycardia pacing |
46 (15.1) |
16 (9.6) |
30 (21.7) |
0.003 |
|
Inappropriate ICD shock |
25 (8.2) |
11 (6.6) |
14 (10.1) |
0.260 |
|
Duration (first shock after device implantation), day |
878 ± 608 |
863 ± 568 |
896 ± 655 |
0.630 |
|
All-cause death |
55 (18.0) |
34 (20.4) |
21 (15.2) |
0.384 |
|
Cardiac |
35 (11.5) |
21 (12.6) |
14 (10.1) |
|
|
Sudden |
5 (1.6) |
2 (1.2) |
3 (2.2) |
|
|
Non-sudden |
30 (9.8) |
19 (11.4) |
11 (8.0) |
|
Non-cardiac |
17 (5.6) |
12 (7.2) |
5 (3.6) |
|
Unknown |
3 (1.0) |
1 (0.6) |
2 (1.4) |
Fig. 2
Appropriate ICD shock and therapy free survivals according to the implantation indication (primary vs. secondary) and etiology (ischemic vs. non-ischemic CMP).
ICD = implantable cardioverter-defibrillator, CMP = cardiomyopathy.
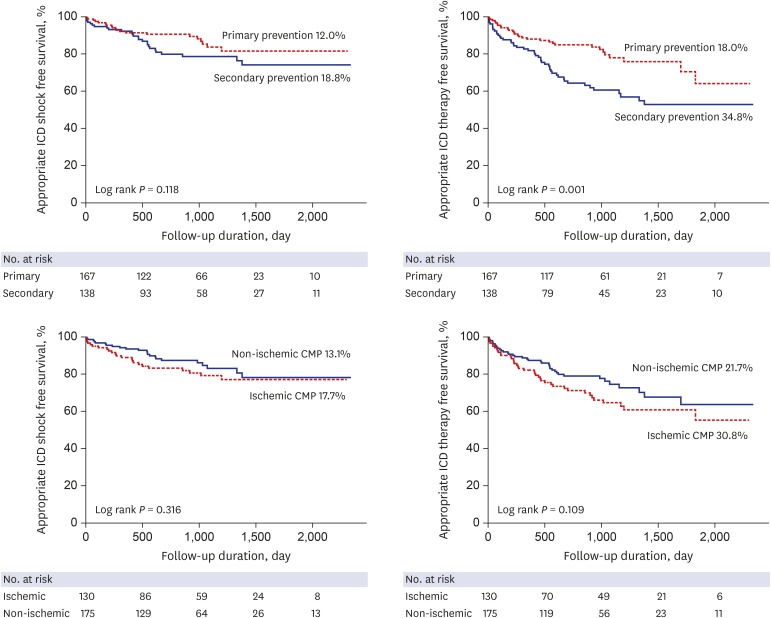
Fig. 3
Kaplan-Meier curve showing survival rate according to the indication and etiology of ICD implantation.
ICD = implantable cardioverter-defibrillator, CMP = cardiomyopathy.
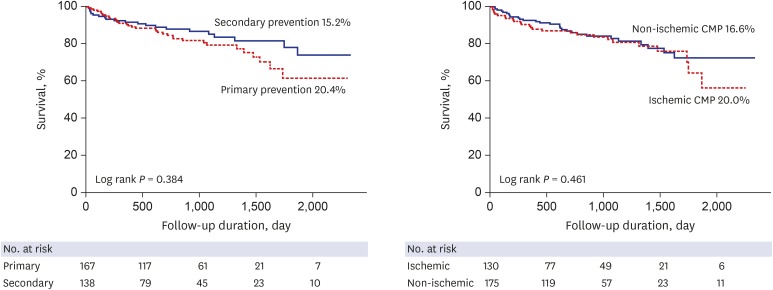
Table 3
Predictors of all-cause death
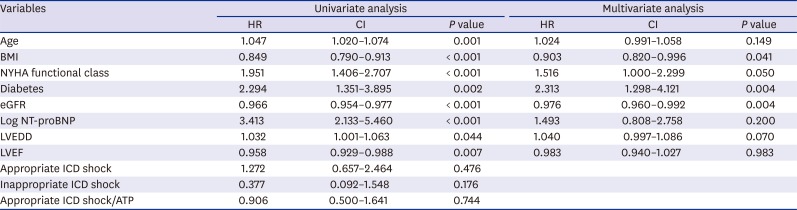
|
Variables |
Univariate analysis |
Multivariate analysis |
|
HR |
CI |
P value |
HR |
CI |
P value |
|
Age |
1.047 |
1.020–1.074 |
0.001 |
1.024 |
0.991–1.058 |
0.149 |
|
BMI |
0.849 |
0.790–0.913 |
< 0.001 |
0.903 |
0.820–0.996 |
0.041 |
|
NYHA functional class |
1.951 |
1.406–2.707 |
< 0.001 |
1.516 |
1.000–2.299 |
0.050 |
|
Diabetes |
2.294 |
1.351–3.895 |
0.002 |
2.313 |
1.298–4.121 |
0.004 |
|
eGFR |
0.966 |
0.954–0.977 |
< 0.001 |
0.976 |
0.960–0.992 |
0.004 |
|
Log NT-proBNP |
3.413 |
2.133–5.460 |
< 0.001 |
1.493 |
0.808–2.758 |
0.200 |
|
LVEDD |
1.032 |
1.001–1.063 |
0.044 |
1.040 |
0.997–1.086 |
0.070 |
|
LVEF |
0.958 |
0.929–0.988 |
0.007 |
0.983 |
0.940–1.027 |
0.983 |
|
Appropriate ICD shock |
1.272 |
0.657–2.464 |
0.476 |
|
|
|
|
Inappropriate ICD shock |
0.377 |
0.092–1.548 |
0.176 |
|
|
|
|
Appropriate ICD shock/ATP |
0.906 |
0.500–1.641 |
0.744 |
|
|
|
Among 167 patients with ICD therapy for primary prevention, 26 patients died before appropriate ICD shock. Level of log NT-proBNP (3.5 ± 0.4 vs. 3.2 ± 0.6,
P = 0.026) and NYHA functional class (2.8 ± 0.8 vs. 2.4 ± 0.7,
P = 0.012) were significantly higher, but eGFR (56 ± 16.3 mL/min vs. 70 ± 27.0 mL/min,
P = 0.001) and BMI (22 ± 3.1 kg/m
2 vs. 25 ± 4.0 kg/m
2,
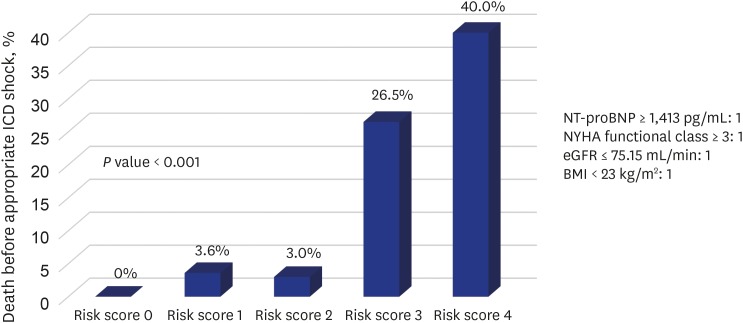
P = 0.003) were significantly lower in patients with death before appropriate ICD shock. The best cut-off levels of NT-proBNP, NYHA functional class, eGFR, and BMI by ROC curve analysis were 1,415 pg/mL, 3, 75.15 mL/min, and 23 kg/m
2, respectively. When patients were categorized in 5 risk score groups according to the sum of values defined by each cut-off level, significant differences in death rate before appropriate ICD shock were observed among risk 0 (0%), 1 (3.6%), 2 (3%), 3 (26.5%), and 4 (40%) (
P < 0.001) (
Fig. 4). In addition, the sum of risk factors was a strong predictor for death before appropriate ICD shock (HR, 2.864; 95% CI, 1.799–4.559;
P < 0.001).
Fig. 4
Death rate before appropriate ICD shock categorized according to the cut-off levels of NT-proBNP, NYHA functional class, eGFR, and BMI. Significant differences in death rate before appropriate ICD shock were observed among risk groups 0 (0%), 1 (3.6%), 2 (3%), 3 (26.5%), and 4 (40%) (P < 0.001).
ICD = implantable cardioverter-defibrillator, NT-proBNP = N-terminal pro-B-type natriuretic peptide, NYHA = New York Heart Association, eGFR = estimated glomerular filtration rate, BMI = body mass index.
DISCUSSION
In our multicenter study, appropriate ICD therapy and shock occurred in 25.6%, and 15.1% of overall patients, respectively. Although appropriate ICD shock rate was not significantly different between primary and secondary prevention groups, appropriate ICD therapy including shock and ATP was more frequently done in the secondary prevention group. Also, indication (primary vs. secondary), etiology (ischemic vs. non-ischemic) and presence or absence of appropriate and/or inappropriate ICD shock did not affect all-cause mortality. Among the primary prevention group, patients with a greater number of poor prognostic factors of heart failure died more often before appropriate ICD shock.
The effect of ICD therapy in patients who have previously experienced VF/hemodynamically unstable VT is well proven.
123 Also, many previous large-scale studies have shown that ICD therapy is beneficial for prevention of SCD in HF patients with decreased LV systolic function.
456 However, the effectiveness of ICD therapy for primary prevention has been based on studies mainly conducted in Western countries and considering the differences in body mass, accompanying disease, and race, it is difficult to apply these findings directly to Asians. Some Korean ICD studies had reported that the frequency of appropriate ICD therapies was not low compared to prior randomized Western trials.
89 However, the previous two Korean ICD studies were conducted in single centers and included only a small number of primary prevention patients despite the long inclusion period. As previously published, our regional multicenter studies on the efficacy of ICD therapy have also had limitations in demonstrating efficacy of ICD therapy for primary prevention due to small number of patients.
10 In the present study, of the 167 primary prevention patients, 20 (12%) experienced appropriate ICD shock, which was not statistically different from that in secondary prevention. Compared to appropriate ICD shock rates in previous primary prevention trials (MADIT-II,
5 14.1% for 21 months, DEFINITE,
4 18% for 29 months, SCD-HeFT,
6 21% for 46 months), the appropriate ICD shock rate of our multicenter registry (12% for 30 months) was similar, or seemed to be lower. However, this means that more than a few patients still had fatal arrhythmias even though most primary prevention patients are taking the guideline-directed management which is mandated by the government. In this regard, our study suggested that ICD therapy for primary prevention is useful.
Many patients who need an ICD for primary prevention have a poor general condition,
1112 and in addition, the various adverse events such as lead or pocket infection, cardiac tamponade, and inappropriate ICD shock
13141516 that develop during or after ICD implantation may be fatal in these patients, and some patients die before experiencing an appropriate ICD shock. Because of this concern, current guidelines recommend ICD therapy for primary prevention in patients who meet the somewhat obscure criteria of life expectancy of more than one year.
7 Of 167 patients with ICD therapy for primary prevention in our study, 26 patients died before appropriate ICD shock. So we analyzed predictors of patients who died before appropriate ICD shock and who did not benefit from primary prevention. NT-proBNP, NYHA functional class, eGFR, and BMI
17181920 are well known as prognostic factors in patients with heart failure and are commonly used in clinical practice. In our multicenter ICD study, high levels of NT-proBNP and NYHA functional class and low levels of eGFR and BMI were associated with death before appropriate ICD shock in the primary prevention group. In addition, when these factors were combined, there was a significantly different death rate before appropriate ICD shock between the high risk score (26.5%–40%) and low risk score group (0%–3.6%). Therefore, the combination of these risk factors can help to differentiate patients who are not benefiting from ICD therapy for primary prevention.
The present study was limited in that it was retrospective and observational. But we think that this study was sufficient to know the efficacy of ICD therapy in actual clinical practice because our study was conducted in multiple centers. Each center has different ICD programming such as VT/VF detection or therapy zone, and use of ATP therapy. These may have affected the outcomes of this study. Also, some VT terminated by ATP therapy might have been hemodynamically stable and this could overestimate the effects of ICDs. During the follow-up period, we could not provide medication data, including beta-blockers, which could affect arrhythmic events. However, since all patients were on medication at the time of ICD implantation, there was no significant change in the follow-up period.
In conclusion, in this multicenter regional registry, the frequency of appropriate ICD therapy is not low in the primary prevention group. In addition, combination of NT-pro BNP, NYHA functional class, eGFR, and BMI, a classic prognostic marker of heart failure, is useful in risk stratification of patients who are not benefiting from ICD therapy for primary prevention.











 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download