1. Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet. 2010; 376:803–813. PMID:
20816547.

2. Song WJ, Cho SH. Challenges in the management of asthma in the elderly. Allergy Asthma Immunol Res. 2015; 7:431–439. PMID:
26122503.

3. Niewoehner DE, Kleinerman J. Morphologic basis of pulmonary resistance in the human lung and effects of aging. J Appl Physiol. 1974; 36:412–418. PMID:
4820322.

4. Milic-Emili J, Torchio R, D'Angelo E. Closing volume: a reappraisal (1967–2007). Eur J Appl Physiol. 2007; 99:567–583. PMID:
17237952.

5. Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999; 13:197–205. PMID:
10836348.

6. Zein JG, Dweik RA, Comhair SA, Bleecker ER, Moore WC, Peters SP, et al. Asthma is more severe in older adults. PLoS One. 2015; 10:e0133490. PMID:
26200463.

7. Haughney J, Price D, Barnes NC, Virchow JC, Roche N, Chrystyn H. Choosing inhaler devices for people with asthma: current knowledge and outstanding research needs. Respir Med. 2010; 104:1237–1245. PMID:
20472415.

8. Virchow JC, Crompton GK, Dal Negro R, Pedersen S, Magnan A, Seidenberg J, et al. Importance of inhaler devices in the management of airway disease. Respir Med. 2008; 102:10–19. PMID:
17923402.

9. Ahookhosh K, Yaqoubi S, Mohammadpourfard M, Hamishehkar H, Aminfar H. Experimental investigation of aerosol deposition through a realistic respiratory airway replica: an evaluation for MDI and DPI performance. Int J Pharm. 2019; 566:157–172. PMID:
31129343.

10. De Backer W, Devolder A, Poli G, Acerbi D, Monno R, Herpich C, et al. Lung deposition of BDP/formoterol HFA pMDI in healthy volunteers, asthmatic, and COPD patients. J Aerosol Med Pulm Drug Deliv. 2010; 23:137–148. PMID:
20109122.

11. Longest PW, Tian G, Walenga RL, Hindle M. Comparing MDI and DPI aerosol deposition using
in vitro experiments and a new stochastic individual path (SIP) model of the conducting airways. Pharm Res. 2012; 29:1670–1688. PMID:
22290350.
12. Usmani OS. Small airways dysfunction in asthma: evaluation and management to improve asthma control. Allergy Asthma Immunol Res. 2014; 6:376–388. PMID:
25228994.

13. Price DB, Román-Rodríguez M, McQueen RB, Bosnic-Anticevich S, Carter V, Gruffydd-Jones K, et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017; 5:1071–1081.e9. PMID:
28286157.

14. Al-Jahdali H, Ahmed A, Al-Harbi A, Khan M, Baharoon S, Bin Salih S, et al. Improper inhaler technique is associated with poor asthma control and frequent emergency department visits. Allergy Asthma Clin Immunol. 2013; 9:8. PMID:
23510684.

15. Newman SP, Weisz AW, Talaee N, Clarke SW. Improvement of drug delivery with a breath actuated pressurised aerosol for patients with poor inhaler technique. Thorax. 1991; 46:712–716. PMID:
1750017.

16. Tashkin DP. New devices for asthma. J Allergy Clin Immunol. 1998; 101:S409–16. PMID:
9500741.

17. Dolovich MB, Ahrens RC, Hess DR, Anderson P, Dhand R, Rau JL, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005; 127:335–371. PMID:
15654001.
18. Global Initiative for Asthma. Global strategy for asthma management and prevention 2015 [Internet]. [place unknown]: Global Initiative for Asthma;2015. cited 2015 Dec. Available from:
http://www.ginasthma.org.
19. Rand CS, Wise RA. Measuring adherence to asthma medication regimens. Am J Respir Crit Care Med. 1994; 149:S69–76. PMID:
8298770.

20. Huitfeldt B, Hummel J. European Federation of Statisticians in the Pharmaceutical Industry (EFSPI). The draft FDA guideline on non-inferiority clinical trials: a critical review from European pharmaceutical industry statisticians. Pharm Stat. 2011; 10:414–419. PMID:
21932294.

21. Müller V, Gálffy G, Eszes N, Losonczy G, Bizzi A, Nicolini G, et al. Asthma control in patients receiving inhaled corticosteroid and long-acting beta2-agonist fixed combinations. A real-life study comparing dry powder inhalers and a pressurized metered dose inhaler extrafine formulation. BMC Pulm Med. 2011; 11:40. PMID:
21762500.

22. Price D, Small I, Haughney J, Ryan D, Gruffydd-Jones K, Lavorini F, et al. Clinical and cost effectiveness of switching asthma patients from fluticasone-salmeterol to extra-fine particle beclometasone-formoterol: a retrospective matched observational study of real-world patients. Prim Care Respir J. 2013; 22:439–448. PMID:
24186700.

23. Rhee CK, van Boven JF, Yau Ming SW, Park HY, Kim DK, Park HS, et al. Does changing inhaler device impact real-life asthma outcomes? Clinical and economic evaluation. J Allergy Clin Immunol Pract. 2019; 7:934–942. PMID:
30292924.

24. Bodzenta-Lukaszyk A, Buhl R, Balint B, Lomax M, Spooner K, Dissanayake S. Fluticasone/formoterol combination therapy versus budesonide/formoterol for the treatment of asthma: a randomized, controlled, non-inferiority trial of efficacy and safety. J Asthma. 2012; 49:1060–1070. PMID:
23102189.

25. Kim MH, Kim SH, Park SY, Ban GY, Kim JH, Jung JW, et al. Characteristics of adult severe refractory asthma in korea analyzed from the severe asthma registry. Allergy Asthma Immunol Res. 2019; 11:43–54. PMID:
30479076.

26. Postma DS, Brightling C, Baldi S, Van den Berge M, Fabbri LM, Gagnatelli A, et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med. 2019; 7:402–416. PMID:
30876830.

27. Contoli M, Kraft M, Hamid Q, Bousquet J, Rabe KF, Fabbri LM, et al. Do small airway abnormalities characterize asthma phenotypes? In search of proof. Clin Exp Allergy. 2012; 42:1150–1160. PMID:
22805462.

28. Ulambayar B, Lee SH, Yang EM, Ye YM, Park HS. Association between epithelial cytokines and clinical phenotypes of elderly asthma. Allergy Asthma Immunol Res. 2019; 11:79–89. PMID:
30479079.
29. Contoli M, Baraldo S, Marku B, Casolari P, Marwick JA, Turato G, et al. Fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease: 5-year follow-up. J Allergy Clin Immunol. 2010; 125:830–837. PMID:
20227753.

30. Martinez CH, Diaz AA, Meldrum C, Curtis JL, Cooper CB, Pirozzi C, et al. Age and small airway imaging abnormalities in subjects with and without airflow obstruction in SPIROMICS. Am J Respir Crit Care Med. 2017; 195:464–472. PMID:
27564413.

31. Cassino C, Berger KI, Goldring RM, Norman RG, Kammerman S, Ciotoli C, et al. Duration of asthma and physiologic outcomes in elderly nonsmokers. Am J Respir Crit Care Med. 2000; 162:1423–1428. PMID:
11029356.

32. Crane MA, Jenkins CR, Goeman DP, Douglass JA. Inhaler device technique can be improved in older adults through tailored education: findings from a randomised controlled trial. NPJ Prim Care Respir Med. 2014; 24:14034. PMID:
25188403.

33. Lurslurchachai L, Krauskopf K, Roy A, Halm EA, Leventhal H, Wisnivesky JP. Metered dose inhaler technique among inner-city asthmatics and its association with asthma medication adherence. Clin Respir J. 2014; 8:397–403. PMID:
24308876.

34. Geller DE. Comparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhaler. Respir Care. 2005; 50:1313–1321. PMID:
16185367.
35. Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest. 2004; 126:213–219. PMID:
15249465.
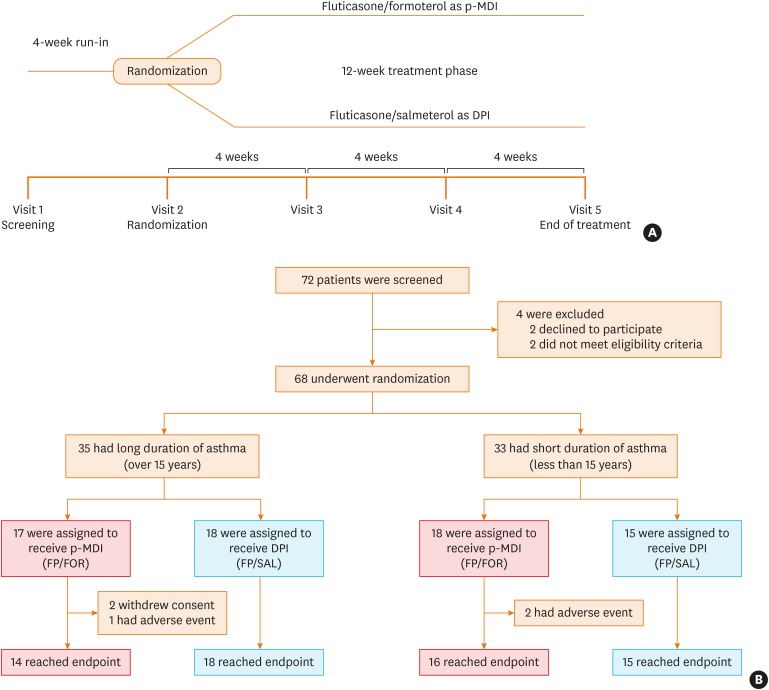
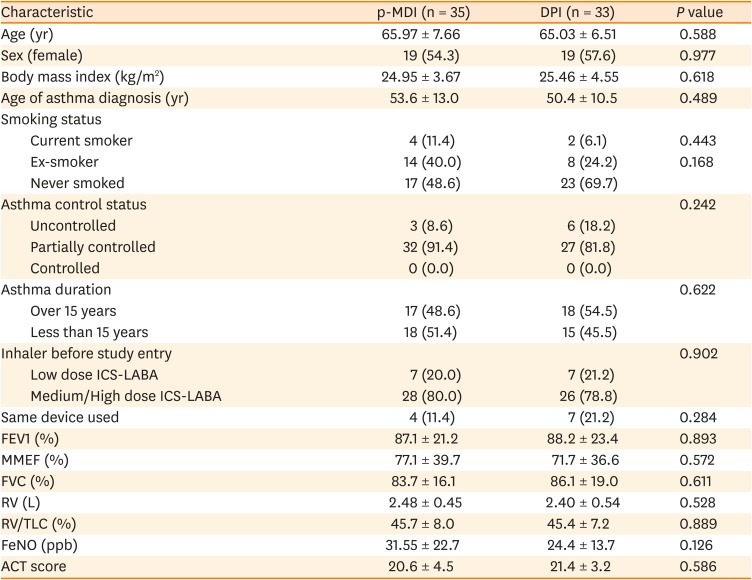
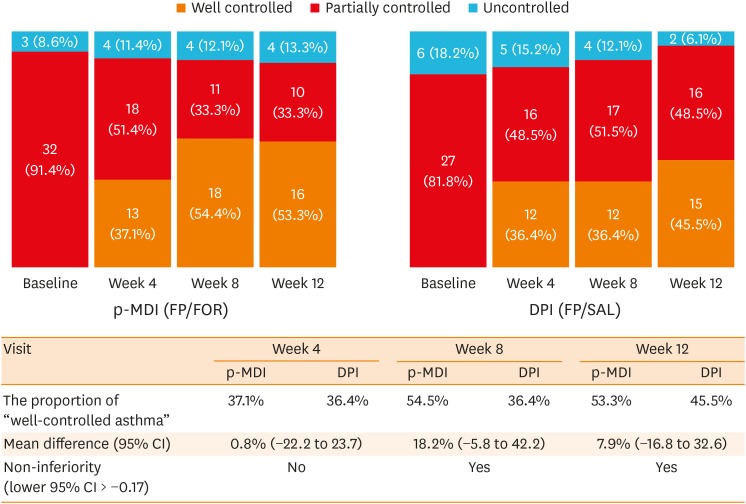
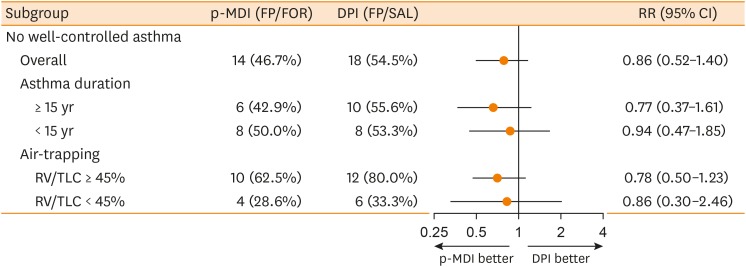




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download