Abstract
Postoperative abdominal adhesion (PAA) causes significant long-term postoperative morbidity. Although numerous physical anti-adhesion barriers (AAB) are used as therapeutical interventions, none of them has achieved sustained success. As a potential strategy to overcome the limitations, drug-eluting AAB have attracted scientific attention. Here, we produced agar films (AF) chemically cross-linked with different concentrations of citric acid (CA) and we measured the physicochemical properties such as crosslinking strength, swelling ratio, hydrophilicity, and biodegradability of the yielded CA-AFs. Next, Metformin (MET), an anti-diabetic drug with anti-proliferative and anti-inflammatory properties, was loaded in the CA-AFs yielding the MET-loaded CA-AF (MET@CA-AF) and the time-dependent MET release was monitored. Based on their physicochemical properties, MET@CA-AF containing 20% CA appeared a promising AAB candidate and was further used in an in vivo study. Mouse models of PAA were established with cecum abrasion and the MET@CA-AF and CA-AF were applied between the injured interfaces. At postoperative day 14, the therapeutic efficacies were analyzed by using clinical adhesion scoring and quantification of collagen-I and fibroblasts in adhesion interfaces. The results showed that applications of MET@CA-AF or CA-AF for 14 days significantly attenuated the clinical adhesion score and thickness of adhesion interface. Furthermore, when compared with the group with operation, the groups with MET@CA-AF or CA-AF exhibited the significant attenuation in PAA-associated myofibroblast activation in adhesion interface. Importantly, these attenuations were significantly more intensified in the group with MET@CA-AF than in the group with CA-AF. Based on our data, we anticipate that MET@CA-AF, a novel synthesized drug-eluting AAB, can protect against PAA by exerting the dual role of physical barrier and MET-based pharmaceutic.
References
1. Tabibian N, Swehli E, Boyd A, Umbreen A, Tabibian JH. Abdominal adhesions: a practical review of an often overlooked entity. Ann Med Surg (Lond). 2017; 15:9–13.

2. Van Goor H. Consequences and complications of peritoneal adhesions. Colorectal Dis. 2007; 9:25–34.

3. Cohen Z, Senagore AJ, Dayton MT, Koruda MJ, Beck DE, Wolff BG, et al. Prevention of postoperative abdominal adhesions by a novel, glycerol/sodium hyaluronate/carboxymeth-ylcellulose-based bioresorbable membrane: a prospective, randomized, evaluator-blinded multicenter study. Dis Colon Rectum. 2005; 48:1130–9.

4. Tabchouri N, Dussart D, Giger-Pabst U, Michot N, Marques F, Khalfallah M, et al. Only surgical treatment to be considered for adhesive small bowel obstruction: a new paradigm. Gastroenterol Res Pract. 2018; 2018:9628490.

5. Lin LX, Yuan F, Zhang HH, Liao NN, Luo JW, Sun YL. Evaluation of surgical anti-adhesion products to reduce postsurgical intraabdominal adhesion formation in a rat model. PLoS One. 2017; 12:e0172088.

6. Yang Y, Liu X, Li Y, Wang Y, Bao C, Chen Y, et al. A postoperative anti-adhesion barrier based on photoinduced imine-crosslinking hydrogel with tissue-adhesive ability. Acta Biomater. 2017; 62:199–209.

7. Wu J, Li P, Dong C, Jiang H, Bin X, Gao X, et al. Rationally designed synthetic protein hydrogels with predictable mechanical properties. Nat Commun. 2018; 9:620.

8. Lee CH, Kim H, Han IW, Kim SM, Kwak BS, Baik YH, et al. Effect of polylactic film (Surgi-Wrap) on preventing postoperative ileus after major hepato-pancreato-biliary surgery. Ann Hepatobiliary Pancreat Surg. 2016; 20:191–6.

9. Rocca A, Aprea G, Surfaro G, Amato M, Giuliani A, Paccone M, et al. Prevention and treatment of peritoneal adhesions in patients affected by vascular diseases following surgery: a review of the literature. Open Med (Wars). 2016; 11:106–14.

10. Lang R, Baumann P, Schmoor C, Odermatt EK, Wente MN, Jauch KW. A-Part Gel, an adhesion prophylaxis for abdominal surgery: a randomized controlled phase I-II safety study [NCT00646412]. Ann Surg Innov Res. 2015; 9:5.

11. Yeo Y, Kohane DS. Polymers in the prevention of peritoneal adhesions. Eur J Pharm Biopharm. 2008; 68:57–66.

12. Maciver AH, McCall MD, Edgar RL, Thiesen AL, Bigam DL, Churchill TA, et al. Sirolimus drug-eluting, hydrogel-im-pregnated polypropylene mesh reduces intraabdominal adhesion formation in a mouse model. Surgery. 2011; 150:907–15.

13. Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008; 49:1993–2007.

14. Leonelli F, La Bella A, Migneco LM, Bettolo RM. Design, synthesis and applications of hyaluronic acid-paclitaxel bio-conjugates. Molecules. 2008; 13:360–78.

15. Chowdhury SM, Hubbell JA. Adhesion prevention with ancrod released via a tissue-adherent hydrogel. J Surd Res. 1996; 61:58–64.

16. Grolman JM, Singh M, Mooney DJ, Eriksson E, Nuutila K. Antibiotic-containing agarose hydrogel for wound and burn care. J Burn Care Res. 2019; 40:900–6.

17. Colquhoun AJ, Venier NA, Vandersluis AD, Besla R, Sugar LM, Kiss A, et al. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis. 2012; 15:346–52.

18. Al-Dwairi A, Alqudah M, Al-Shboul O, Alfaqih M, Alo-mari D. Metformin exerts antiinflammatory effects on mouse colon smooth muscle cells in vitro. Exp Ther Med. 2018; 16:985–92.
19. Zucca P, Fernandez-Lafuente R, Sanjust E. Agarose and its derivatives as supports for enzyme immobilization. Molecules. 2016; 21:E1577.

20. Wong RSH, Dodou K. Effect of drug loading method and drug physicochemical properties on the material and drug release properties of poly (Ethylene Oxide) hydrogels for transdermal delivery. Polymers (Basel). 2017; 9:E286.

21. Council NR. Guide for the care and use of laboratory animals. 8th ed.Washington, DC: The National Academies Press;2011.
22. Gao Q, Wei G, Wu Y, Yao N, Zhou C, Wang K, et al. Paeoni-florin prevents postoperative peritoneal adhesion formation in an experimental rat model. Oncotarget. 2017; 8:93899–911.

23. Lauder CI, Garcea G, Strickland A, Maddern GJ. Use of a modified chitosan-dextran gel to prevent peritoneal adhesions in a rat model. J Surg Res. 2011; 171:877–82.

24. Jeong JH, Kim DK, Lee NS, Jeong YG, Kim HW, Kim JS, et al. Neuroprotective effect of nortriptyline in overt hepatic encephalopathy through attenuation of mitochondrial dysfunction. ASN Neuro. 2018; 10:1759091418810583.

25. Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH protoc. 2008; 2008:pdb. .prot4986.

26. Wu Q, Wang N, He T, Shang J, Song L, Yang X, et al. Ther-mosensitive hydrogel containing dexamethasone micelles for preventing postsurgical adhesion in a repeated-injury model. Sci Rep. 2015; 5:13553.

27. Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. J Adv Res. 2015; 6:105–21.

28. Moris D, Chakedis J, Rahnemai-Azar AA, Wilson A, Hennessy MM, Athanasiou A, et al. Postoperative abdominal adhesions: clinical significance and advances in prevention and management. J Gastrointest Surg. 2017; 21:1713–22.

29. Rajab TK, Ahmad UN, Kelly E. Implications of late complications from adhesions for preoperative informed consent. J R Soc Med. 2010; 103:317–21.
30. Hosseini A, Akhavan S, Menshaei M, Feizi A. Effects of streptokinase and normal saline on the incidence of intraabdominal adhesion 1 week and 1 month after laparotomy in rats. Adv Biomed Res. 2018; 7:16.

31. Charboneau AJ, Delaney JP, Beilman G. Fucoidans inhibit the formation of postoperative abdominal adhesions in a rat model. PLoS One. 2018; 13:e0207797.

33. Rouhani A, Tabrizi A, Ghavidel E. Effects of nonsteroidal antiinflammatory drugs on flexor tendon rehabilitation after repair. Arch Bone Jt Surg. 2013; 1:28–30.
34. Rosenberg SM, Board JA. High-molecular weight dextran in human infertility surgery. Am J Obstet Gynecol. 1984; 148:380–5.

35. Topal E, Ozturk E, Sen G, Yerci O, Yilmazlar T. A comparison of three fibrinolytic agents in prevention of intra-abdominal adhesions. Acta Chir Belg. 2010; 110:71–5.

36. Molinas CR, Binda MM, Manavella GD, Koninckx PR. Adhesion formation after laparoscopic surgery: what do we know about the role of the peritoneal environment? Facts Views Vis Obgyn. 2010; 2:149–60.
37. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010; 123:4195–200.

38. Kanno Y. The role of fibrinolytic regulators in vascular dysfunction of systemic sclerosis. Int J Mol Sci. 2019; 20:E619.

39. Irkorucu O, Ferahköşe Z, Memiş L, Ekinci O, Akin M. Reduction of postsurgical adhesions in a rat model: a comparative study. Clinics (Sao Paulo). 2009; 64:143–8.
40. Thornton MH, Johns DB, Campeau JD, Hoehler F, DiZerega GS. Clinical evaluation of 0.5% ferric hyaluronate adhesion prevention gel for the reduction of adhesions following peritoneal cavity surgery: open-label pilot study. Hum Reprod. 1998; 13:1480–5.

41. Park IH, Um JY, Hong SM, Cho JS, Lee SH, Lee SH, et al. Metformin reduces TGF-β1-induced extracellular matrix production in nasal polyp-derived fibroblasts. Otolaryngol Head Neck Surg. 2014; 150:148–53.

42. Sato N, Takasaka N, Yoshida M, Tsubouchi K, Minagawa S, Araya J, et al. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir Res. 2016; 17:107.

43. Dehkordi AH, Abbaszadeh A, Mir S, Hasanvand A. Metformin and its antiinflammatory and antioxidative effects; new concepts. J Renal Inj Prev. 2019; 8:54–61.

Fig. 1.
The novel synthesized MET@CA-AF. (A) The macroscopic appearance of MET@CA-AF. (B) Chemical formula of MET@CA-AFs after cross-linking reaction and the predicted releasing dynamics of MET from MET@CA-AF.
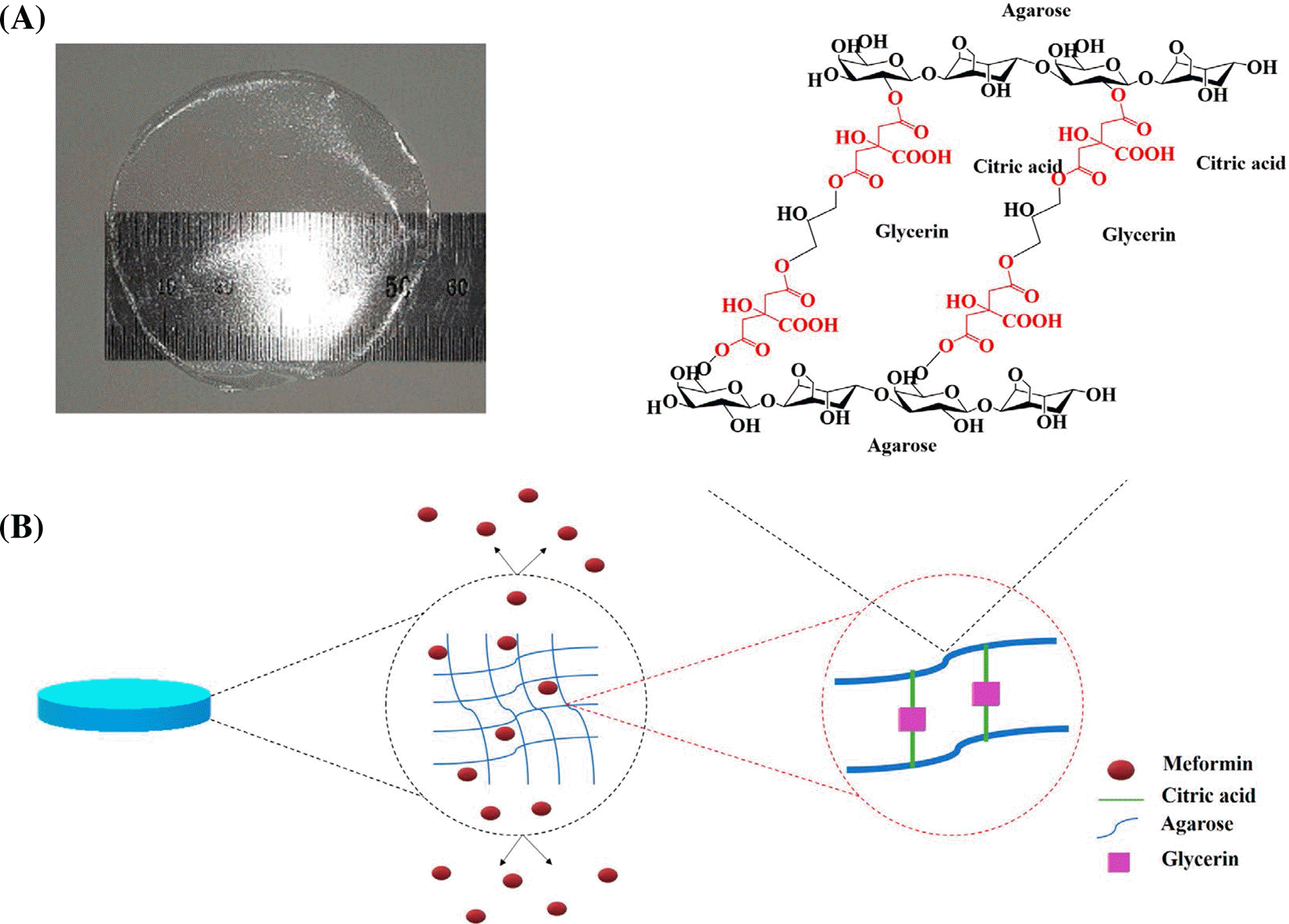
Fig. 2.
Physicochemical properties of the CA-AFs with different concentrations of CA and MET@CA-AF. (A) FT-IR spectra with indicat-ed esterification, (B) swelling properties, (C) in vitro degradation properties, (D) hydrophilicity, and (E) in vitro MET-releasing properties of CA-AFs cross-linked with different concentrations of CA. In graphs of (B) and (D), data were represented as mean±S.D (p∗∗∗<0.001 between the indicated samples).
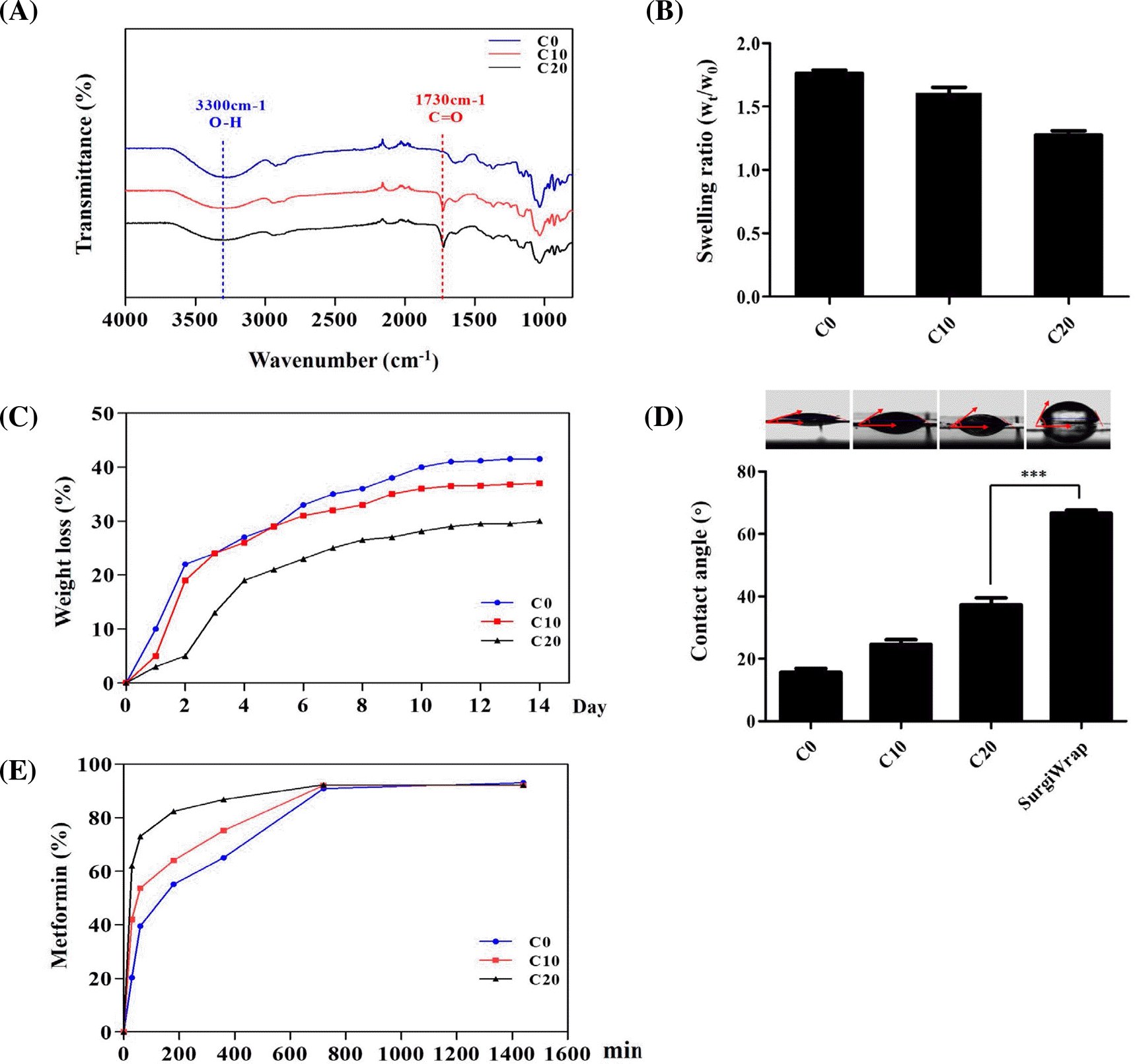
Fig. 3.
Attenuation of gross findings associated with postoperative abdominal adhesion by MET@CA-AF. (A) Representative laparoscopic images obtained at POD 14. The wide and well-developed adhesions are indicated with black arrowheads, the abdominal wall is indicated as AW, and the cecum is indicated as C. (B) H-E stained tissue samples involving the adhesive lesion. Scale bar = 100 μm.
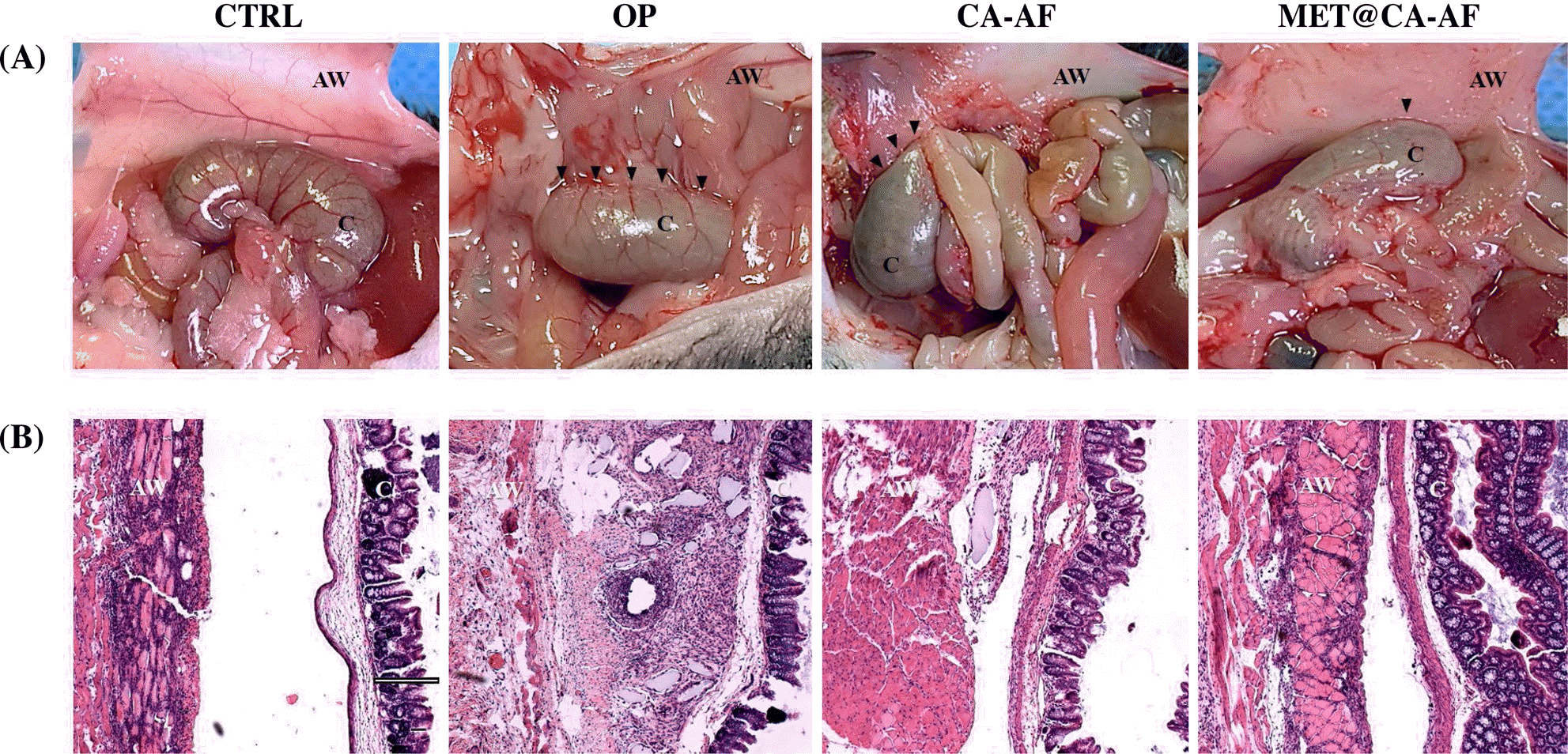
Fig. 4.
Inhibition of the adhesion interface formation and myofibroblast activation by MET@CA-AF. (A) Representative Masson's tri-chrome-stained tissue samples involving the adhesive lesion. Scale bar = 100 μm. (B) Quantitative graphs showing the averaged thicknesses of adhesion interface of different groups. Data are represented as mean± S.D (p∗∗∗<0.001 vs. OP group. p†††<0.001 vs. CA-AF group). (C) Representative images of immunofluorescence for detecting activated myofibroblast, which is depicted as DAPI+ vimentin+ cells surround-ed by collagen-I+ fluorescence (indicated with white arrows). Scale bar = 50 μm. (D) Quantitative graphs showing the average number of activated myofibroblasts of different groups. Data are represented as mean± S.D (p∗∗<0.01 and p∗∗∗<0.001 vs. OP group; p††<0.01 vs. CA-AF group).
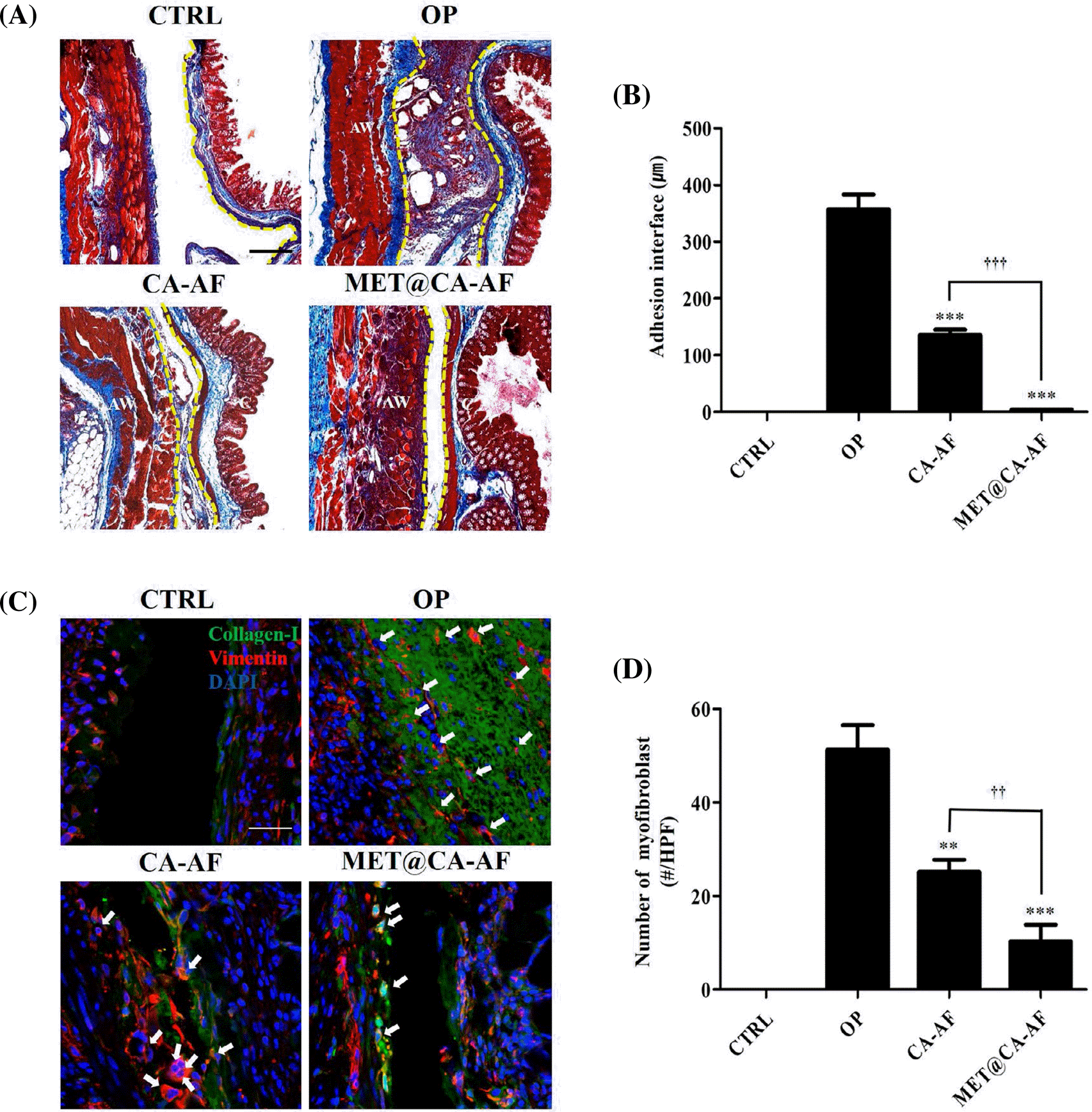
Table 1.
Attenuation of PAA severity by MET@CA-AF. Adhesions are graded from 0 to 5: 0, no adhesion; 1, one thin filmy adhesion; 2, more than one thin adhesion; 3, thick adhesion with focal point; 4, thick adhesion with planar attachment or more than one thick adhesion with focal point; and 5, very thick vascularized adhesion or more than one planar adhesion. Data are represented as a mean± S.D.
| Adhesion | CTRL | OP | CA-AF | MET@CA-AF |
|---|---|---|---|---|
| 0 | 8 | 0 | 0 | 4 |
| 1 | 0 | 0 | 1 | 2 |
| 2 | 0 | 0 | 2 | 0 |
| 3 | 0 | 0 | 3 | 0 |
| 4 | 0 | 3 | 0 | 0 |
| 5 | 0 | 2 | 0 | 0 |
| Death | 0 | 3 | 2 | 2 |
| Mean±S.D | 0 | 4.4±0.55 | 2.3±0.82∗∗∗ | 0.33±0.52∗∗∗,††† |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download