INTRODUCTION
When administering dental treatment to adult patients with severe intellectual disabilities, it is generally difficult to ensure an appropriate cooperation level by behavioral management, and general anesthesia (GA) is therefore performed for effective treatment [
1]. However, patients with disabilities who show indications for GA may present difficulties during GA induction due to a lack of cooperation [
2]. In particular, it has been reported that when treating adult patients with cooperation impairment accompanied by physical disabilities, autism, Down syndrome or epilepsy, physical restraint was needed in over 30% of such patients during anesthesia induction [
3]. Moreover, in many cases, preoperative patient evaluation and lab tests cannot be performed properly, and severe needle phobia makes it often difficult to secure an intravenous (IV) line for anesthesia induction [
2]. Unlike in pediatric patients, physical restraint in adult patients with a healthy physique may threaten the safety of the operator or patient, cause postoperative mental suffering, and lead to ethical issues [
4].
Premedication is a method typically used to alleviate patient anxiety prior to GA or surgery [
5]. In patients with intellectual disabilities, the use of midazolam [
6], ketamine [
7], and dexmedetomidine [
8] has been reported to increase levels of preanesthesia cooperation. However, the use of intramuscular (IM) injections or nasal sprays may present yet another challenge in adult patients who show violent behavior, and oral administration is thus preferred [
6], which offers the advantage of inducing positive patient behavior prior to anesthesia, since there is no discomfort for the patient. However, administration of oral sedatives in people with disabilities who are uncooperative bears the risk of respiratory depression and hypoxia due to overdosing or pulmonary aspiration due to vomiting [
910], while it also has the disadvantages of a slow onset, unpredictability of effects, and possible delays in postoperative recovery [
11].
Midazolam is commonly used as a premedication drug for behavior management prior to GA in patients with disabilities [
12]. The Special Care Clinic at Seoul National University Dental Hospital (SNUDH) has been performing oral premedication with midazolam when patients with disabilities show severe cooperation impairments. Midazolam is administered orally, 1 or 2 tablets depending on the body weight of the patient. After 20–30 min, when a state of sedation is reached, anesthesia induction can be initiated. However, there may be some patients who do not show adequate sedation, due to their pharmacological characteristics, while there are other cases when additional IM injection of ketamine is needed because the patient could not be sedated at all or GA is performed while someone forcefully restrains the patient [
13].
Unfortunately, since 2013, oral midazolam is no longer commercially available in South Korea. As an alternative to midazolam, SNUDH chose triazolam, which is commonly used for reducing anxiety during dental treatment [
14], and since 2014, 2–3 tablets of oral triazolam have been administered as premedication prior to anesthesia induction in adult patients with disabilities who have difficulties cooperating.
While the authors were able to use triazolam as premedication to achieve a cooperation level similar to that achieved with midazolam, reports on the effect of triazolam as premedication prior to anesthesia in adult patients with disabilities who show cooperation difficulties could not be found.
The present study therefore retrospectively analyzed anesthesia and recovery room records of adult patients with disabilities who underwent GA or deep sedation with oral administration of midazolam or triazolam between 2009 and 2017, for the purpose of assessing the premedication effect of triazolam. Patient cooperation levels during anesthesia induction, administered doses, anesthesia duration, and length of recovery room stay were investigated, and the differences between oral midazolam and triazolam were analyzed.
Go to :

MATERIALS AND METHODS
The present study was approved by the Institutional Review Board of Seoul National University School of Dentistry (IRB No. S-02018014). The SNUDH medical information database was searched for patients who were prescribed oral midazolam or triazolam for dental treatment under GA at the Special Care Clinic between January 1, 2009 and December 31, 2017. From the resulting list, only the patients whose anesthesia and recovery room records were available and those who underwent GA or deep sedation after actually taking the sedative that was prescribed were included in the study. Preanesthesia evaluation, anesthesia records, and recovery room records of the selected patients were analyzed (
Fig. 1), and type of disability, reason for prescribing the oral sedative, administered drug and dose, cooperation level during anesthesia induction, anesthesia duration, and length of recovery room stay were assessed.
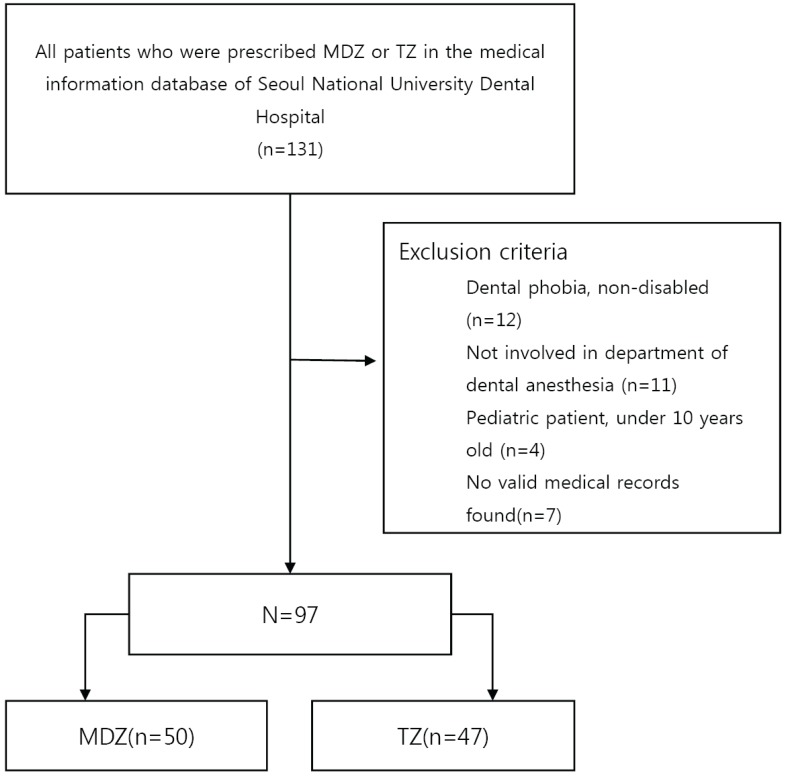 | Fig. 1A flow chart of the study population is presented (MDZ = midazolam, TZ = triazolam).
|
All dental patients with disabilities included in the study period had undergone outpatient GA or deep sedation according to the treatment guidelines of the SNUDH Special Care Clinic. Preanesthesia evaluation, which included history taking, physical examinations, and laboratory tests, was performed through an outpatient visit made at least 2 weeks prior to the planned treatment date. Moreover, the patients were given advance notice on preparations they needed to make prior to anesthesia. On the day of anesthesia, the patients were required to fast for at least 8 h and, subsequently, anesthesia was performed after verifying that there was no exacerbation of any preexisting condition and that the patient did not suffer from other conditions such as a cold. Explanation was given to the guardian on the GA or deep sedation procedure and potential complications, particularly regarding the possibility of using physical restraint, oral administration of triazolam, or IM injections of ketamine if the patient refuses to come into the dental office or shows violent behavior. After providing these explanations, signed informed consent was obtained.
Prior to anesthesia induction, if the patient refused to come into the dental office for GA or showed severely negative behavior, midazolam (Dormicum Tab. 7.5 mg, Roche Inc., Switzerland) or triazolam (Halcion Tab. 0.25 mg, Pfizer Inc., USA) was used for premedication after explaining the situation to the guardian. In the patient waiting room, midazolam 7.5–22.5 mg (1–3 tablets) or triazolam 0.25–0.75 mg (1–3 tablets), depending on the body weight of the patient, was administered orally with about 100 ml of water. After observing the patient for about 30–40 min, anesthesia induction was initiated. If the patient became unconscious, they were moved to the dental chair and, while measuring the vital signs, intravenous anesthetics were administered after intravenous catheter insertion or sevoflurane induction was performed. If the patient did not respond at all or very little to oral midazolam or triazolam, anesthesia induction was performed under physical restraint without any additional drug administration. If the patient vigorously refused, 5 mg/kg of ketamine were administered via IM injection, and anesthesia was induced after the patient lost consciousness. In other words, the appropriate method for anesthesia induction was selected according to how each patient responded. The anesthesia records contained information on the anesthesia induction process, the patient's response over time after midazolam or triazolam administration, and their cooperation level. As shown in
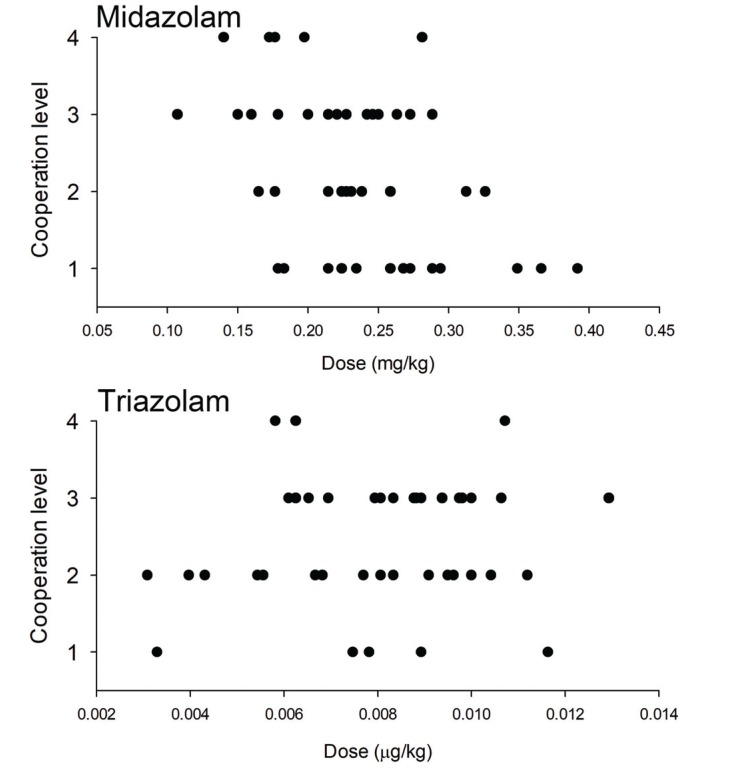
Table 1, cooperation was assessed on 4 levels and recorded accordingly [
3]. Upon completion of GA or deep sedation, the patients were transferred to the recovery room, monitored until they regained complete consciousness, and when they were able to walk out on their own they were given an explanation on precautions and discharged.
Table 1
Cooperation level during dental general anesthesia induction

|
Cooperation level |
Description |
|
Level 1 |
Willing to receive the anesthetic induction |
|
Level 2 |
Performed without much problem despite some resistance |
|
Level 3 |
Performed after physical restraint since cooperation was not possible |
|
Level 4 |
Performed using other methods, such as IM injection of ketamine, due to the patient being very violent |

For each item investigated, results are presented as the number of patients or the mean value plus standard deviation, as well as maximum and minimum values. Where necessary, results are presented as percentages. During medical history taking, if records were missing or incomplete, only valid data were used in the analysis. Statistical analyses were performed using SPSS 25 (IBM Inc, USA); paired t-tests and chi-square tests were conducted, and a P-value < 0.05 was considered statistically significant.
Go to :

DISCUSSION
Performing anesthesia induction when bringing adult patients with disabilities who refuse to cooperate into the dental office room represents a major challenge for anesthesiologists. Nonetheless, performing GA on adult patients with disabilities in order to complete all possible treatments within a single round of anesthesia, instead of multiple rounds of treatments, can be very beneficial to the patients. Successful anesthesia induction can help form a favorable relationship between the patient and dentist, since treatment under anesthesia does not induce dental phobia in the patient. Moreover, because the patient does not move, anesthesia enables high quality treatment and reduces stress in the operator and patient [
15].
To perform anesthesia induction in patients who strongly refuse anesthesia, various methods are being used, including comforting the patient, administering premedication, and using physical restraint. During the process of physically restraining the patient in unavoidable situations, the patient or the medical staff attempting anesthesia may be injured. Moreover, difficulties with airway maintenance may occur during anesthesia, and there is a high likelihood of the patient being exposed to possible anesthesia complications, such as hyper- or hypotension [
16]. Forced restraint poses a risk of mental shock to the patient, and when the guardian of the patient witnesses such restraint, the relationship between the guardian and doctor may be compromised or legal problems may arise.
However, patients with intellectual disabilities have experience with physical restraint or abuse and have difficulties with situation awareness; as a result, there is controversy about the occurrence of stress syndrome in such patients [
17]. However, studies have reported that patients with brain injury who were forcibly restrained during treatment responded more poorly during their subsequent treatments [
18] and that patients who struggled during anesthesia induction tended to show slower recovery [
19] and an increased frequency of excitatory responses in their daily life [
20].
Because of these reasons, increasing the patient cooperation level by using premedication is preferred over using physical restraint. A review of articles on the effects of premedication using preanesthesia sedatives showed that premedication is mostly used for reducing preanesthesia anxiety in pediatric patients, while reports on its use in adult patients with disabilities are difficult to find. Premedication that can be used generally include oral administration of midazolam, IM injection of ketamine, and administration of dexmedetomidine [
21]. However, oral administration is preferred over IV or IM injection, since it has a higher patient compliance. Among the sedatives that can be administered orally, fast-acting drugs with a short half-life that can be used during anesthesia induction are limited to just midazolam and triazolam. Some studies have reported on oral administration of ketamine [
7] or dexmedetomidine [
8], which is typically administered by IV injections, in adult patients with disabilities, but in South Korea, these 2 drugs are approved for injection purposes only, and thus, oral administration of these drug has legal ramifications.
Benzodiazepines can reduce preanesthesia anxiety and improve cooperation levels during anesthesia induction owing to their sedative effect and, in addition, their amnesic effect can help block unpleasant memories formed during anesthesia induction from being recalled. In particular, after oral administration of midazolam, a sedative effect appears within 20–30 min. A study that orally administered midazolam syrup as premedication prior to GA in pediatric patients showed that the effect of administering 0.25 mg/kg was equivalent to that of higher doses of 0.5 and 1.0 mg/kg [
22]. In pediatric patients, the maximum effect generally appears within 20–30 min after oral administration, and the effect is sustained for 90 min. However, the effect may appear from 10 min after oral administration in some cases [
23]. Moreover, since the effect begins to disappear after 45 min, additional drug administration may be necessary. Although midazolam can be administered by an intranasal route, the patient may experience this as unpleasant.
Generally, the purpose of premedication using sedatives such as midazolam is to reduce anxiety about anesthesia and surgery, and not to induce lower consciousness. However, most studies that used premedication for the purpose of inducing lower consciousness did so to control behavior in pediatric patients with no cognitive impairment. Therefore, it is difficult to use such findings to set an appropriate dose for adult patients with disabilities. In particular, overdoses of oral sedatives in patients with cerebral palsy or brain lesions puts them at risk of respiratory depression. A study on premedication for dental treatment in adult patients with disabilities reported that when midazolam was administered by IM injection (0.15 mg/kg) and orally (0.3 mg/kg), oral administration was more effective than IM administration [
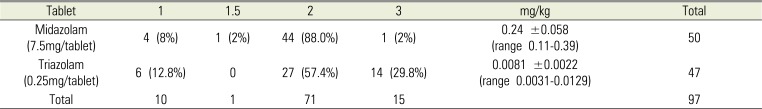
6]. Because the doses of most studies were used for premedication in pediatric patients, using them as a reference for adult patients with disabilities would be questionable. However, based on years of clinical use of midazolam, the present study reveals that 2 tablets of midazolam are generally used for premedication, and the post-hoc analysis results show that on average, 0.24 mg/kg of midazolam are administered.
Triazolam is a benzodiazepine class oral sedative with fast onset and action, which is often used for treating insomnia. In addition to its hypnotic effect, it also has amnesic, anti-anxiety, sedative, and anticonvulsant effects, and it is effective in reducing anxiety during dental treatment [
14]. Moreover, oral administration prior to dental IV sedation is known to significantly reduce the recall of discomfort felt during IV catheter insertion [
24]. Generally, the drug is sold in 0.25-mg tablets and administered orally or sublingually. With an oral administration, the maximum effect appears in 1 h, while the elimination half-life is known to be 1.5–5.5 h. Overdoses may cause complications such as respiratory depression, coma, and seizures, especially in patients with cerebral palsy or brain lesions, but recovery is possible by gradual injection of flumazenil. For oral administration, South Asians show higher peak blood concentrations than Caucasians, while their time to reach peak concentrations is also known to be shorter with 45 min (30–75 min) [
25].
Studies on triazolam dosage in healthy adults reported that when 0.25-, 0.5-, and 0.75-mg doses of triazolam were administered sublingually, peak BIS depression was reached after 80 min with a single 0.25-mg dose, while gradual administration of 0.5 and 0.75 mg showed a maximum effect being reached at 67 ± 14 and 60 ± 16 min, respectively [
26]. Since the study population in the present study consisted of adult patients with intellectual disabilities, it would be difficult to directly apply the results mentioned above. However, analyses of years of clinical data showed that 0.5–0.75 mg was administered, which indicates a dose distribution that is similar to the one in the studies mentioned above. Because the drug is available in tablet form, it was administered according to the body weight of each patient, but it was difficult to estimate the appropriate dose. In the present study, anesthesia induction may have been performed slightly early with respect to the effect onset time of the drug, but the analysis of the patient's responses according to time after oral administration did not show noticeable differences.
In the triazolam group, flumazenil was used in 3 cases, all of which involved patients with severe intellectual disabilities. Two of the patients had received 3 tablets of triazolam, whereas the other patient, weighing 40 kg, had received 2 tablet. In all 3 cases, the operating time was short, within about 1 h, and an IV injection of 0.5 mg of flumazenil was given due to breathing depression after extubation. In all 3 cases, the patients were discharged after staying in the recovery room for about 1 h.
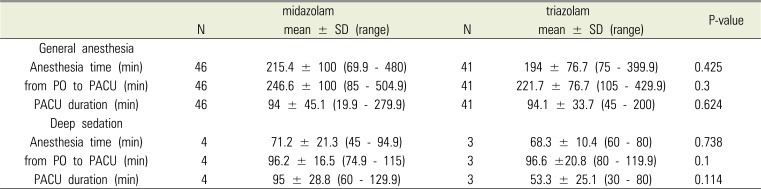
In conclusion, using oral sedatives as premedication in adult patients with disabilities and cooperation difficulties can allow anesthesia induction without any physical restraint in approximately 50% of all cases, while a comparison between triazolam and midazolam showed no significant difference. In addition, there were no significant differences in time to anesthesia induction and recovery time between the 2 groups.
The present study retrospectively reviewed patient medical records. Although detailed records of patient responses before and after drug administration were available, there were some missing data. Moreover, because the study was not conducted with the study population divided by dose groups, it may be difficult to use the current findings for determining the appropriate administration dose.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download