Abstract
Background
Third molar extraction is associated with considerable pain and discomfort, which is mostly managed with oral analgesic medication. We assessed the analgesic effect of benzydamine hydrochloride, a topical analgesic oral rinse, for controlling postoperative pain following third molar extraction.
Methods
A randomized controlled trial was conducted in 40 patients divided into two groups, for extraction of fully erupted third molar. Groups A received benzydamine hydrochloride mouthwash and group B received normal saline gargle with oral ibuprofen and paracetamol. Oral ibuprofen and paracetamol was the rescue analgesic drug in group A. Patients were evaluated on the 3rd and 7th post-operative days (POD) for pain using the visual analogue score (VAS), trismus, total number of analgesics consumed, and satisfaction level of patients.
Results
The VAS in groups A and B on POD3 and POD7 was 4.55 ± 2.54 and 3.95 ± 1.8, and 1.2 ± 1.64 and 0.95 ± 1.14, respectively and was statistically insignificant. The number of analgesics consumed in groups A and B on POD3 (5.25 ± 2.22 and 6.05 ± 2.43) was not statistically different from that consumed on POD7 (9.15 ± 5.93 and 10.65 ± 6.46). The p values for trismus on POD3 and POD7 were 0.609 and 0.490, respectively and those for patient satisfaction level on POD3 and POD7 were 0.283 and 0.217, respectively.
Third molar extraction, one of the most common oral surgeries performed, is associated with considerable pain and discomfort. Numerous studies have been conducted to identify optimal analgesics for the relief of pain and discomfort following third molar extraction. The analgesic efficacy of COX 2 inhibitors, including celecoxib, valdecoxib and non-steroidal anti-inflammatory drugs (NSAID), such as ibuprofen, and opioids, such as oxycodone have been studied for pain management in oral surgery [12]. Preemptive analgesia with dexamethasone alone or co-administered with either ibuprofen or diclofenac sodium were investigated [34]. Different formulations of the same medication, such as diclofenac sodium softgel were also investigated [5]. All studies have shown promising results but these studies used only oral medications for pain control. Adverse effects of NSAID medication, which is the mainstay of treatment for third molar extraction surgery, might be varied. These effects include nausea, gastritis, gastro intestinal hemorrhage, analgesic nephropathy, and coagulation defect. Local anesthetics, including bupivacaine and lignocaine hydrochloride with methyl prednisolone have been shown to reduce post-operative pain and swelling following third molar extraction. However, only the intraoperative conditions were observed [67]. Reduced consumption of topical morphine or ketamine after tonsillectomy has been reported [8]. No definitive outcomes have been reported with regards to the use of oral rinses, mouthwashes, and sprays for recovery after post tonsillectomy pain [9].
Topical administration of NSAIDs might help in reducing side effects without actually decreasing the quality of analgesia. Benzydamine hydrochloride, a topical NSAID, has been studied and found to be effective for treating oral mucositis and oral ulcers, as well as for the prevention of post-operative sore throat following endotracheal intubation [101112]. Amidst the plethora of studies not much has been experimented with the use of topical oral analgesic rinse in third molar extraction surgery. We hypothesized that analgesic mouth rinses can reduce the use of oral analgesics, thus reducing the side effects associated with oral analgesic use without compromising the quality of analgesia. In this study, our aim was to assess analgesic requirement and patient satisfaction after using topical oral analgesic benzydamine hydrochloride mouthwash and after using only oral analgesics.
A randomized controlled trial was conducted in a tertiary care specialized oral and maxillofacial unit. As this was a pilot study, each group comprised 40 cases. This study followed the Declaration of Helsinki on medical protocol and ethics, and was approved by the Institute Ethical Review Board the study vide Institutes ethical review board no. IEC/NP-377/08.10.2014, RP-16/2014. It was registered in Clinical trials registry – India with reference no. CTRI/2017/08/009220.
Only patients with the American Society of Anesthesiologist physical status I & II coming for fully erupted mandibular third molar extraction were included in the study. Patients with tooth requiring surgical extraction, periodontally weak tooth, tooth associated with periapical pathology, tooth associated with fracture, tooth with cellulitis or abscess, and tooth where the adjacent 2nd molar may be a source of pain (e.g. due to caries) were excluded from the study. Patients with other conditions that may alter the healing capacity post-extraction, such as diabetes mellitus and those receiving bisphosphonate therapy or post radiotherapy were also excluded.
Randomization was performed using a sequence of random numbers generated by the RALLOC software. The two intervention mouth-rinses bottles were numbered as per the random number table. Allocation concealment was performed using the “SNOSE technique.” The random number table was kept with the administrative head.
The patient and operator were not blinded, however the person collecting the post-operative data was blinded. Patients could not be blinded because benzydamine hydrochloride is sweet, slightly stinging in taste with a peppermint odor, and has a clear yellowish-green color and normal saline is clear and salty in taste. The operator could not be blinded because the two liquids have different colors and odors. The person writing the prescription was not involved in the further study.
Once the patient was enrolled in the study, two minutes before the extraction he/she was given a mouth wash labeled A or B as per randomization and thereafter, extraction was performed by a single experienced surgeon who has performed more than 1000 extractions. The inferior alveolar, long buccal branch of mandibular, and lingual nerves were blocked during the extraction.
Group A was the intervention group which received 0.15 % w/v of benzydamine hydrochloride mouthwash while group B (the control group) patients received only normal saline gargles. All the patients received short-term antibiotic (Amoxycillin 500 mg TDS × 3 days), and an oral dose comprising 400 mg of ibuprofen and 500 mg of paracetamol one hour post-extraction. This dose was administered every eight hours for a period of 24 hours.
From the second day onwards, group A patients did not receive any analgesics but performed gargles with benzydamine hydrochloride only while group B patients continued to receive oral analgesics comprising 400 mg of ibuprofen and 500 mg of paracetamol three times a day with normal saline gargle. The oral dose comprising 400 mg of ibuprofen and 500 mg of paracetamol was the rescue drug in group A. Patients received this oral dose if their VAS was > 4 and this was recorded in the form provided. VAS was explained to the patient beforehand.
All the patients were instructed to brush their teeth three times per day post-extraction for five days and advised to do gargles as prescribed from the 2nd post-extraction day five to six times per day for five days. Patients recorded the number of times brushing was done per day and use of gargles in the prescribed form which was provided.
Base-line parameters: age (years), sex, reason for extraction (periodontitis, caries, or prophylactic), side of extraction, oral hygiene status (measured using the OHI-S index) [13], time taken for extraction (< 5 minutes, 5–15 minutes, > 15 minutes), and any intra-operative complications were noted. After the extraction, difficulty index was assessed based on a modified Parant's index [14] for surgical extraction. Only those patients who fulfilled the criteria for the Type 1 (Easy) category of the Parant's index were included in the study.
Patients performed self-evaluation at home. At the hospital, they were evaluated on the 3rd and 7th post-extraction day. Pain was assessed using the VAS. Trismus was evaluated by using a simple grading system. In this system, a score of 0 was assigned where trismus was absent, 1 was assigned when mouth opening was > 76% of normal, 2 was assigned when mouth opening was less than 75% and more than 51% of normal, 3 was assigned when mouth opening was less than 50% but more than 26% of normal, and 4 was assigned when mouth opening was less than 25% of normal. The total number of analgesics consumed was calculated. The satisfaction level of patient on Global assessment of pain was done on a 5-point categorical scale; 0 being poor satisfaction level, 1 being fair, 2 being good satisfaction level, 3 as very good and 4 as excellent scores. Any complication occurring within the study period was also recorded.
A written consent for publication was obtained from all patients after enrollment in the study.
Statistical analysis was performed with the SPSS statistical software version 15.0 (IBM corporation, Chicago; IL, USA). Continuous variables were analyzed with the Student's t test while categorical variables were analyzed using the Pearson's chi-square test. The Wilcoxon rank sum test was used to compare nonnominal data. P values of < 0.05 were considered to be statistically significant.
A total of 40 patients fulfilling the inclusion criteria were enrolled in the study. Demographic characteristics of both groups were comparable. Details are summarized in Table 1.
Although the ages of patients in both groups are statistically different, they are essentially all adults and belong to a similar physiological age range. Therefore, the age should not have any influence on the outcome of the study.
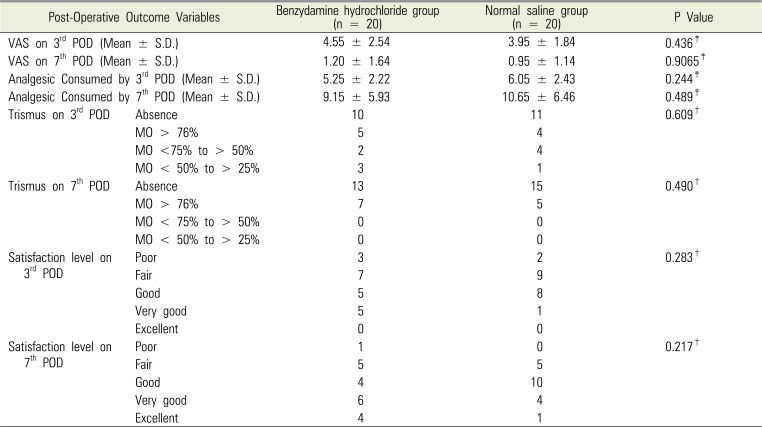
Dental carries was the single largest cause for third molar extraction, followed by prophylactic extraction and periodontitis. The reasons for extraction were similar in both groups with no statistical difference (P = 0.88). The time taken for extraction was less than 5 mins in majority of the cases with no significant statistical difference between the groups (P = 0.376). The OHI-S index was comparable in both groups (P = 0.376). Pain scores assessed by VAS determined the need for oral analgesics. VAS on the 3rd and 7th PODs in group A was higher than that for group B but the difference was statistically insignificant (P = 0.436 and P = 0.9065, respectively). Patients who used benzydamine hydrochloride oral rinse consumed less oral analgesics (5.25 ± 2.2, mean ± SD) as compared to patients who used normal saline gargle (6.05 ± 2.4, mean ± SD) on the 3rd POD and 9.15 ± 5.9 Versus 10.65 ± 6.5 respectively on 7th POD, but the difference was statistically insignificant (P = 0.244 and P = 0.489, respectively). Comparison of trismus in both groups did not yield any statistical difference either on the 3rd or 7th POD (P = 0.609 and P = 0.490, respectively). Similarly, patient satisfaction level was comparable in both groups on both 3rd and 7th PODs (P = 0.28 and P = 0.22, respectively). Thus, in summary, topical NSAID benzydamine hydrochloride did reduce the use of oral analgesic consumption as compared to the use of normal saline gargle but statistically the difference was insignificant. The satisfaction level after using benzydamine hydrochloride was same as that after using normal saline gargle as shown in Table 2.
Pain following third molar extraction is a subject of several studies all in the quest of finding the optimal analgesia. Oral analgesics, either NSAIDS or opioids, have been used for the purpose. Although a favorable satisfaction level has been demonstrated with the use of diclofenac, celecoxib, and rofecoxib, there are potential complications when taken orally. Significant information could not be found in literature on the use of oral topical analgesic rinses.
Benzydamine hydrochloride {1-benzyl-3-[3(dimethylamino)-propoxy]-1H-indazole hydrochloride} is a non-steroidal drug with anti-inflammatory, analgesic, and anti-microbial properties. The drug is believed to exert its effect by forming thromboxanes which decrease prostaglandin production leading to the stabilization of cell membranes and inhibition of platelet aggregation [15].
Studies involving topical application of analgesics, such as morphine for oral mucositis in children [16], and morphine and ketamine for post tonsillectomy pain [8], have shown decrease in pain scores and less analgesic consumption in the postoperative period.
After comparing oral nimesulide with benzydamine hydrochloride oral rinses for inflammatory ear, nose, and throat diseases, it was shown that nimesulide had a more rapid effect and higher patient tolerability [17].
Fedorowicz Z et al. reviewed studies on oral rinses, mouthwashes, and sprays for improving recovery following tonsillectomy but could not achieve a definitive outcome [9]. Sini and Ivan Djordjevi, compared benzydamine hydrochloride and salvia officinalis (artificial saliva) as adjuvant local treatments with systemic nonsteroidal anti-Inflammatory drugs [18]. In their study, which involved clinical trials on children and adults who underwent tonsillectomy, adenoidectomy, or both, benzydamine hydrochloride was more effective than saliva officinalis in controlling postoperative pain and infection. The effect of benzydamine hydrochloride on pain relief was faster and persisted for one week after surgery. The safety profiles of both the formulations were the same. Benzydamine hydrochloride was more efficacious in preventing postoperative infections in adults [18].
In our study, we did not observe the benefits of topical benzydamine hydrochloride as demonstrated above. It is plausible that the drug works in adenotonsillectomy because the raw area along with the nerve endings are exposed allowing the topical medication to act. In mandibular 3rd molar extraction, however, the wound is deep and often covered by a blood clot. Thus, the raw area remains inaccessible to the topical drug. G. Roopashri et al. conducted a drug trial on the efficacy of benzydamine hydrochloride, chlorhexidine, and povidone iodine in the treatment of oral mucositis among patients undergoing radiotherapy. They observed that benzydamine hydrochloride was more efficient in the management of radiation-induced mucositis [10].
Agarwal A et al. evaluated the efficacy of aspirin and benzydamine hydrochloride gargle in attenuating postoperative sore throat (POST) and they concluded that aspirin and benzydamine hydrochloride gargles were effective in significantly reducing the incidence and severity of POST [12]. Benzydamine hydrochloride produced a more prolonged effect than aspirin. Diclofenac mouthwash has been evaluated for treating inflammatory conditions of the mouth and for periodontal surgery, and has been shown to be effective [192021]. A limitation of this study is that the study, being a pilot study, has a small sample size. The difference in age groups could have been eliminated by a larger sample size. Another limitation as that, since patients and the operator could not be blinded due to the taste and color of normal saline and benzydamine hydrochloride solutions, there may have been some bias.
In conclusion, benzydamine hydrochloride oral rinses reduce the intake of oral analgesics in an insignificant manner and is inadequate for pain relief following mandibular third molar extraction.
ACKNOWLEDGEMENTS
Dr Krushna Bhat, Assistant Professor, supervised the project and helped in statistical analysis.
Notes
References
1. Cheung R, Krishnaswami S, Kowalski K. Analgesic efficacy of celecoxib in post-operative oral surgery pain: a single-dose, two-center, randomized, double-blind, active- and placebo-controlled study. Clin Ther. 2007; 29(Suppl):2498–2510. PMID: 18164917.

2. Daniels SE, Desjardins PJ, Talwalker S, Recker DP, Verburg KM. The analgesic efficacy of valdecoxib vs. oxycodone/acetaminophen after oral surgery. J Am Dent Assoc. 2002; 133:611–621. PMID: 12036167.

3. Bauer HC, Duarte FL, Horliana AC, Tortamano IP, Perez FE, Simone JL, et al. Assessment of preemptive analgesia with ibuprofen coadministered or not with dexamethasone in third molar surgery: a randomized double-blind controlled clinical trial. Oral Maxillofac Surg. 2013; 17:165–171. PMID: 22949122.

4. Shah R, Mahajan A, Shah N, Dadhania AP. Preemptive analgesia in third molar impaction surgery. Natl J Maxillofac Surg. 2012; 3:144–147. PMID: 23833488.

5. Zuniga JR, Phillips CL, Shugars D, Lyon JA, Peroutka SJ, Swarbrick J, Bon C. Analgesic safety and efficacy of diclofenac sodium softgels on postoperative third molar extraction pain. J Oral Maxillofac Surg. 2004; 62:806–815. PMID: 15218558.

6. Christensen J, Matzen LH, Vaeth M, Wenzel A, Schou S. Efficiency of bupivacaine versus lidocaine and methylprednisolone versus placebo to reduce postoperative pain and swelling after surgical removal of mandibular third molars: a randomized, double-blinded, crossover clinical trial. J Oral Maxillofac Surg. 2013; 71:1490–1499. PMID: 23866780.

7. Satya Bhushan NV, Nayak RN. A comparison of the efficacy of topical application of lignocaine hydrochloride 5% gel and bupivacaine hydrochloride 5% gel for extraction of teeth. J Maxillofac Oral Surg. 2010; 9:119–126. PMID: 22190770.

8. Canbay O, Celebi N, Uzun S, Sahin A, Celiker V, Aypar U. Topical ketamine and morphine for post-tonsillectomy pain. Eur J Anaesthesiol. 2008; 25:287–292. PMID: 18186954.

9. Fedorowicz Z, van Zuuren EJ, Nasser M, Carter B, Al Langawi JH. Oral rinses, mouthwashes and sprays for improving recovery following tonsillectomy. Cochrane Database Syst Rev. 2013; 10:CD007806.

10. Roopashri G, Jayanthi K, Guruprasad R. Efficacy of benzydamine hydrochloride, chlorhexidine, and povidone iodine in the treatment of oral mucositis among patients undergoing radiotherapy in head and neck malignancies: a drug trail. Contemp Clin Dent. 2011; 2:8–12. PMID: 22114446.

11. Karavana SY, Güneri P, Ertan G. Benzydamine hydrochloride buccalbioadhesive gels designed for oral ulcers: preparation, rheological, textural, mucoadhesive and release properties. Pharm Dev Technol. 2009; 14:623–631. PMID: 19883251.
12. Agarwal A, Nath SS, Goswami D, Gupta D, Dhiraaj S, Singh PK. An evaluation of the efficacy of aspirin and benzydamine hydrochloride gargle for attenuating postoperative sore throat: a prospective, randomized, single-blind study. Anesth Analg. 2006; 103:1001–1003. PMID: 17000820.

13. Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964; 68:7–13. PMID: 14076341.

14. Garcia AG, Sampedro FG, Rey JG, Vila PG, Martin MS. Pell-Gregory classification is unreliable as a predictor of difficulty in extracting impacted lower third molars. Br J Oral Maxillofac Surg. 2000; 38:585–587. PMID: 11092770.
15. Sardella A, Uglietti D, Demarosi F, Lodi G, Bez C, Carrassi A. Benzydamine hydrochloride oral rinses in management of burning mouth syndrome. a clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 88:683–686. PMID: 10625850.
16. Nielsen BN, Aagaard G, Henneberg SW, Schmiegelow K, Hansen SH, Romsing J. Topical morphine for oral mucositis in children: dose finding and absorption. J Pain Symptom Manage. 2012; 44:117–123. PMID: 22658469.

17. Milvio C. Nimesulide for the treatment of painful inflammatory process in the ear, nose and throat areas: a double-blind controlled study with benzydamine. J Int Med Res. 1984; 12:327–332. PMID: 6519348.

18. Lalićević S, Djordjević I. Comparison of benzydamine hydrochloride and salvia officinalis as an adjuvant local treatment to systemic nonsteroidal anti-inflammatory drug in controlling pain after tonsillectomy, adenoidectomy, or both: an open-label, single-blind, randomized clinical trial. Curr Ther Res Clin Exp. 2004; 65:360–372. PMID: 24672091.
19. Serafini G, Trevisan S, Saponati G, Bandettini B. Therapeutic efficacy and tolerability of the topical treatment of inflammatory conditions of the oral cavity with a mouthwash containing diclofenace polamine: a randomized, investigator-blind, parallel-group, controlled, phase III study. Clin Drug Investig. 2012; 32:41–49.
20. Tramèr M, Bassetti C, Metzler C, Morgantini A. Efficacy and safety of mouthwash diclofenac in oral or periodontal surgery. Minerva Stomatol. 2001; 50:309–314. PMID: 11723430.
21. Weinstein RL. Double blind placebo-controlled study on efficacy, acceptability and safety of mouthwash diclofenac in oral or periodontal postoperative period. Minerva Stomatol. 2001; 50:315–319. PMID: 11723431.
Table 1
Comparison of base line variables in the benzydamine hydrochloride oral rinse and normal saline oral rinse groups
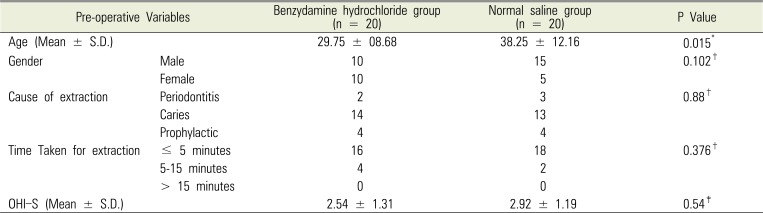
Table 2
Comparison of outcome parameters between the benzydamine hydrochloride and normal saline group




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download