This article has been
cited by other articles in ScienceCentral.
Abstract
Paresthesia is an altered sensation of the skin, manifesting as numbness, partial loss of local sensitivity, burning, or tingling. The inferior alveolar nerve (IAN) is the third branch of the trigeminal nerve and is very important in dental treatment. IAN paresthesia may occur after various dental procedures such as simple anesthetic injections, surgical procedures, and endodontic treatment, and is reported to range from 0.35% to 8.4%. The altered sensation usually follows immediately after the procedure, and reports of late onset of nerve involvement are rare. This report presents a rare case of delayed paresthesia after dental surgery and discusses the pathophysiology of IAN delayed paresthesia.
Keywords: Delayed Paresthesia, Inferior Alveolar Nerve, Pathophysiology
Paresthesia is an altered sensation of the skin, manifesting as numbness, partial loss of local sensitivity, burning, or tingling [
1]. Facial paresthesia has a known etiology in 83% of cases, and 48% of these have been attributed to a dental procedure [
2]. In paresthesia resulting from dental procedures, the inferior alveolar nerve (IAN) and lingual nerves are the most commonly implicated nerves [
13].
The IAN is the third branch of the trigeminal nerve and is a very important nerve in dental treatment. After branching off from the trigeminal nerve, the IAN enters the mandibular foramen of the mandibular ramus and travels to the mandibular molars. After this nerve exits the mental foramen of the mandible, it controls sensation of the lower teeth, lips, chin, and cheek [
4]. IAN paresthesia may occur after various dental procedures such as simple anesthetic injections, surgical procedures, and endodontic treatment, and can manifest as altered sensation to the lips, skin of the cheek and chin, tongue, intraoral mucosa, and teeth [
5].
IAN paresthesia occurs in 0.35% to 8.4% of patients, and the neurologic symptom duration varies greatly from days or weeks to several months [
67]. In general, neurosensory deficits after third molar surgery spontaneously recover in the first 6 postoperative months and the incidence of permanent sensory disturbance was reported as 0.12% [
68]. Direct trauma to the IAN during dental procedures and indirect trauma from edema or hematoma are reported mechanisms of IAN paresthesia [
19].
The altered sensation is usually noted by the patient on the day of surgery, once the effects of any local anesthetic have resolved [
579]. However, on rare occasions, patients report onset of paresthesia a few days to months after the procedure [
1011121314]. Delayed paresthesia was represented by only 5% of the 60 cases of paresthesia reported in a study of 1477 third molar surgeries [
15]. The biggest difference between classic paresthesia and delayed paresthesia is that the former begins immediately after the procedure and healing is not guaranteed, while the latter occurs later, with restoration to original condition [
10].
Here we present a rare case of delayed paresthesia after dental surgery and discuss the pathophysiology of IAN-related delayed paresthesia.
CASE REPORT
A 53-year-old woman presented to the department of Advanced General Dentistry at Dankook University College of Dentistry in 2017 for dental treatment. She reported occasional discomfort of the left lower third molar, and her medical history was otherwise unremarkable.
The patient had slight swelling of the pericoronal tissue around the left lower third molar. The left lower third molar was very close to the inferior alveolar canal on radiography (
Fig. 1). The patient was diagnosed with chronic pericoronitis and surgical removal was planned. After informed consent was provided by the patient, IAN block anesthesia was performed using two ampoules of 2% lidocaine with 1:100000 epinephrine (Huons, Sungnamsi, Korea). After confirming efficacy of local anesthesia (absence of lip and chin sensation), surgical extraction and suturing were performed. No intraoperative complications were encountered, and the IAN was not visualized during surgery.
The early postoperative period was uneventful. However, 2 weeks postoperatively, the patient returned complaining of recent onset of numbness of her lower lip, chin, and lower teeth on the left side, as well as increased discomfort of the extraction site. She reported no specific precipitating event, but sudden pain at the extraction site and a dulled sensitivity of the left side over the previous 24 h. On clinical examination, the extraction site showed no swelling and redness of soft tissue but partial loss of blood clot was observed (
Fig. 2). Submandibular lymphadenopathy was not present. We performed careful subjective and objective assessments. The patient did not have a pin prick sensation, but reported an overall sensation of dullness. Skin mapping of the affected area showed abnormal sensation in the lower lip and chin approximately 20 mm in width and spreading from the vermilion mucosal border of the lip down to the chin (
Fig. 3). Neurosensory testing was performed according to the recommendations of Poort et al. [
16]. Three sensory tests, including the brush direction test, pin point test based on a visual analog scale, and two-point discrimination test were performed on the left chin with the unaffected right side as a control site [
16]. The results showed that left side sensation was decreased (it was relatively lower than 10 when the control site sensation was 10) and the two-point discrimination test was 15 mm (normal right side was 12 mm) (
Table 1). Postoperative cone beam computed tomography revealed that continuity of the upper cortical layer of the inferior alveolar canal was partially destroyed (
Fig. 4). The patient was diagnosed with delayed paresthesia of IAN, and anti-inflammatory drugs and steroids were prescribed. The patient returned to the clinic one week later. The pain at the extraction site had disappeared, but there was no improvement in the sensory dullness of the lips and chin. The patient was instructed to take the remaining prescription medicines and to come in for follow-up in 1 month. One month later (6 weeks of paresthesia), the paresthesia had resolved completely by both patient report and subjective testing. We finally diagnosed this patient with delayed paresthesia of neuropraxia of IAN.
DISCUSSION
This patient presented with typical symptoms of IAN paresthesia occurring approximately 2 weeks postoperatively. The paresthesia lasted for about 5 weeks, after which the patient reported a complete recovery. We diagnosed this patient with delayed paresthesia by neuropraxia of IAN.
Seddon classified nerve injuries based on the severity of the injury as neurapraxia, axonotmesis, and neurotmesis [
17]. Neurapraxia is the mildest classification of peripheral nerve injury, characterized by a temporary loss of sensory function due to blockage of nerve conduction, usually lasting an average of 6 to 8 weeks before full recovery. This condition is typically caused by a blunt neural injury due to nerve compression in which external pressure causes decreased blood flow to the nerve and deformation of the nerve fibers. Neurapraxia results in temporary damage to the myelin sheath but leaves the nerve (axon) intact and is an impermanent condition. The thinning of the myelin sheath or focal demyelination are the main consequences of the injury that lead to conduction blockage. In order for the condition to be considered neurapraxia, there must be a complete and relatively rapid recovery of sensory function once nerve conduction has been restored; otherwise, the injury would be classified as axonotmesis or neurotmesis [
1819].
Therefore, pressure from surrounding tissue edema may be the pathophysiology of delayed paresthesia. Dahli et al. [
20] showed that rabbit tibial nerves compressed at 50 mmHg for 2 h had normal afferent and motor conduction velocity, whereas the nerves compressed at 200 mmHg for 2 h exhibited reduction of conduction velocity only at the area of compression. At 400 mmHg for 2 h, conduction velocity was reduced both at the level of compression and distal to the compressed segment. Borgonovo et al. [
10] reported three cases of delayed paresthesia after third molar extraction, and considered compression caused by the clot, fibrous organization, and bone fragments as possible etiologies of delayed paresthesia. They indicated that all three may promote inflammation onset along the nerve trunk, and the paresthesia was induced by inflammatory edema [
10]. In our case, however, neuropraxia due to compression is not applicable since the clot was lost rather than organized, and insertion of bone fragments was not observed on cone beam computed tomography.
Other papers have described the pathophysiology of delayed paresthesia from different perspectives.
Flanagan [
11] commented in his article that hemoglobin has been associated with delayed neuropathy. Hemoglobin degrades and liberates iron, which generates free radicals, which in turn degrade type I collagen and other molecules. Neuropathy from a hematoma may be related to the presence of iron compounds in the presence of the involved nerve. Goldberg and Galbraith [
12] commented in their study that the pathophysiologic mechanism of delayed paresthesia may include direct bacterial invasion of the neural sheath or inflammation of the nerve, as well as pressure secondary to the edema of the inflammatory process, as in inflammatory neuritis of peripheral nerves.
In our case, when the patient revisited due to dulled sensation of the left side, she also reported pain at the extraction site in which the soft tissue healing was normal but partial loss of blood clot was observed, similar to alveolar osteitis. Alveolar osteitis is described as postoperative pain originating from the extraction socket, which peaks approximately 1 to 3 days after tooth extraction and is associated with partial or total loss of the blood clot from the socket, with or without halitosis. Clinical and laboratory studies have shown the significance of locally increased fibrinolytic activity in the pathogenesis of alveolar osteitis. Direct tissue activators after trauma and indirect activators produced by bacteria cleave other plasminogen molecules to plasmin, resulting in the breakup of the clot by disintegrating the fibrin [
21]. Therefore, in our case, hemoglobin may be released after fibrinolysis, and it may degrades and liberates iron, generating free radicals. The free radicals may damage the myelin sheath or affect nerve conduction. Actinomyces viscosus, Streptococcus mutans, and anaerobic organisms (also the predominant organisms in pericoronitis) in the alveolar osteitis socket are regarded to have possible significance in the etiology of alveolar osteitis. Therefore, it can be suggested that the delayed paresthesia in our case may be caused by bacterial invasion [
21]. However, there were no other signs of infection such as lymphadenopathy, swelling, or redness of extraction site, and bacterial invasion is unlikely to be a major factor.
In conclusion, the pathophysiology of delayed paresthesia in our patient is thought to be a temporary conduction blockage due to degradation from free radicals in fibrinolysis and, partially, bacterial invasion of the neural sheath.
Figures and Tables
 | Fig. 1Preoperative radiographs demonstrating left lower third molar, which was very close to the inferior alveolar canal. (A) Panoramic radiograph, (B) Cone beam computed tomography: panoramic view, (C) Cone beam computed tomography: cross-sectional view.
|
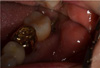 | Fig. 2Two weeks postoperatively (first day of paresthesia), the extraction site showed no swelling or redness of the soft tissue but partial loss of blood clot was observed.
|
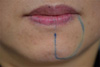 | Fig. 3Skin mapping of the affected area outlining abnormal sensation.
|
 | Fig. 4Postoperative radiographs demonstrating the discontinuity of upper cortical layer of the inferior alveolar canal. (A) Cone beam computed tomography: panoramic view, (B) Cone beam computed tomography: cross-sectional view.
|
Table 1
The results of neurosensory tests performed 14 days after extraction (first day of paresthesia)

|
Right side |
Left side |
|
Brush direction test |
10 |
7 |
|
Pin prick test |
10 |
5 |
|
Two-point discrimination test |
12 mm |
15 mm |







 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download