INTRODUCTION
MATERIALS AND METHODS
1. Inclusion and exclusion criteria
2. Outcomes
3. Search methods for identification of studies
-
MEDLINE via PubMed (searched on 03/22/2017; updated on 04/01/2018) limited to English language and Humans:
(“IANB” OR ((“mandible”[MeSH Terms] OR “mandible” OR “mandibular”) AND “block”[All Fields]) OR (“mandibular nerve”[MeSH Terms] OR (“mandibular” AND “nerve”) OR “mandibular nerve” OR (“inferior” AND “alveolar” AND “nerve”) OR “inferior alveolar nerve”)) AND ((“buffers”[MeSH Terms] OR “buffers” OR “buffered” OR “sodium bicarbonate” OR (sodium AND bicarbonate) OR “alkalinized”) AND (“lidocaine” [MeSH Terms] OR “lidocaine”)) -
The Web of Science (searched on 03/22/2017; updated on 04/01/2018):
TS=((inferior alveolar block OR mandibular block) AND (buffered lidocaine OR (lidocaine AND (buffers OR buffered)))) -
The Cochrane Library (searched on 03/22/2017; updated on 04/01/2018):
(inferior alveolar block or mandibular block) and (buffered lidocaine or (lidocaine and (buffers or buffered))) -
The Embase (searched on 03/22/2017; updated on 04/01/2018):
(‘lidocaine’/exp OR lidocaine) AND (buffer OR buffered) AND ((inferior AND alveolar AND block) OR (mandibular AND block)) -
The ProQuest (searched on 04/01/2018):
(“buffered lidocaine”) AND (“inferior alveolar nerve block”) limited to scholarly journals and dissertations and theses
4. Data collection and analysis
5. Data extraction and management
Table 1
Summary of eligible studies
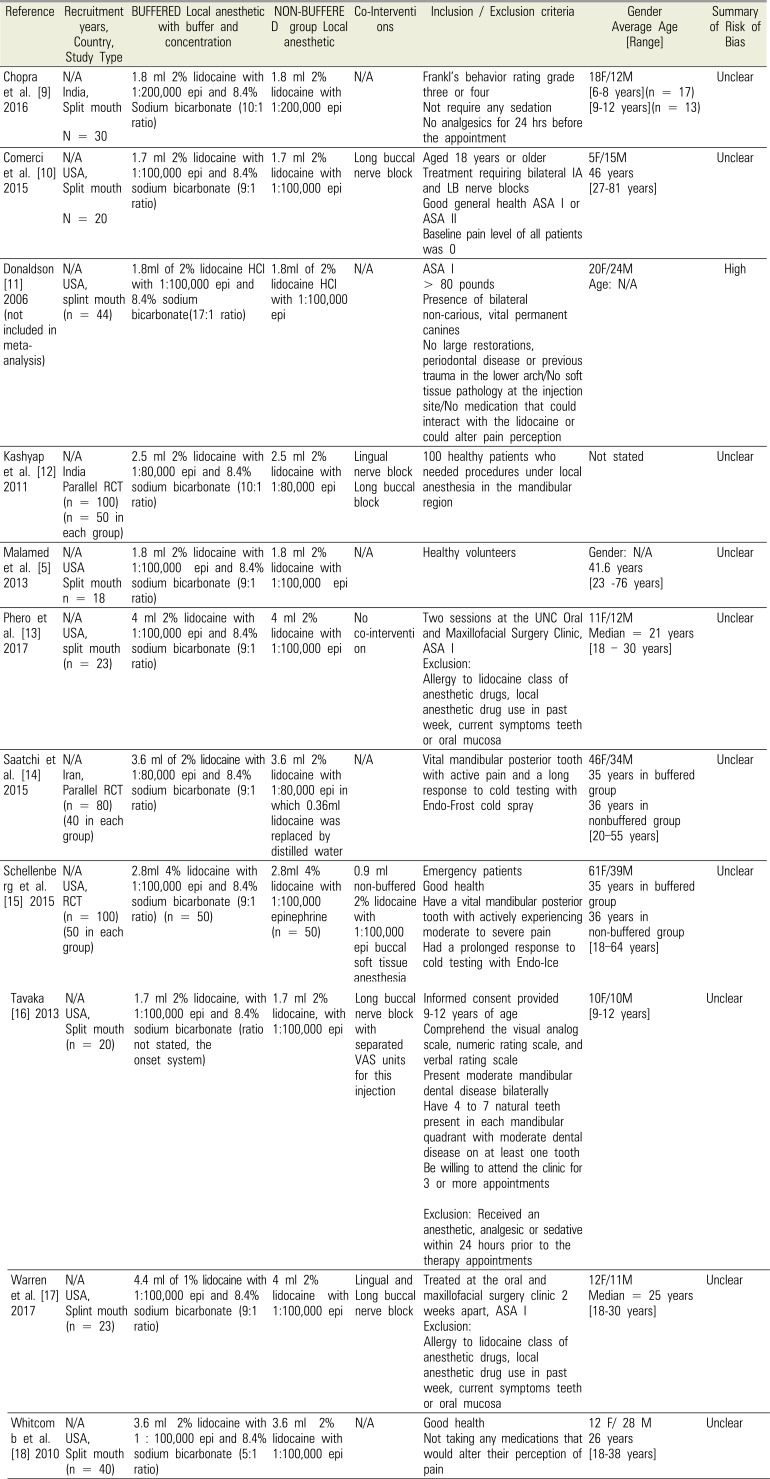
| Reference | Recruitment years, Country, Study Type | BUFFERED Local anesthetic with buffer and concentration | NON-BUFFERE D group Local anesthetic | Co-Interventions | Inclusion / Exclusion criteria | Gender Average Age [Range] | Summary of Risk of Bias |
|---|---|---|---|---|---|---|---|
| Chopra et al. [9] 2016 | N/A | 1.8 ml 2% lidocaine with 1:200,000 epi and 8.4% Sodium bicarbonate (10:1 ratio) | 1.8 ml 2% lidocaine with 1:200,000 epi | N/A | Frankl's behavior rating grade three or four | 18F/12M | Unclear |
| India, | Not require any sedation | [6–8 years](n = 17) | |||||
| Split mouth | No analgesics for 24 hrs before the appointment | [9–12 years](n = 13) | |||||
| N = 30 | |||||||
| Comerci et al. [10] 2015 | N/A | 1.7 ml 2% lidocaine with 1:100,000 epi and 8.4% sodium bicarbonate (9:1 ratio) | 1.7 ml 2% lidocaine with 1:100,000 epi | Long buccal nerve block | Aged 18 years or older | 5F/15M | Unclear |
| USA, | Treatment requiring bilateral IA and LB nerve blocks | 46 years | |||||
| Split mouth | Good general health ASA I or ASA II | [27–81 years] | |||||
| N = 20 | Baseline pain level of all patients was 0 | ||||||
| Donaldson [11] 2006 (not included in meta-analysis) | N/A | 1.8ml of 2% lidocaine HCl with 1:100,000 epi and 8.4% sodium bicarbonate(17:1 ratio) | 1.8ml of 2% lidocaine HCl with 1:100,000 epi | N/A | ASA I | 20F/24M | High |
| USA, | > 80 pounds | Age: N/A | |||||
| splint mouth | Presence of bilateral non-carious, vital permanent canines | ||||||
| (n = 44) | No large restorations, periodontal disease or previous trauma in the lower arch/No soft tissue pathology at the injection site/No medication that could interact with the lidocaine or could alter pain perception | ||||||
| Kashyap et al. [12] 2011 | N/A | 2.5 ml 2% lidocaine with 1:80,000 epi and 8.4% sodium bicarbonate (10:1 ratio) | 2.5 ml 2% lidocaine with 1:80,000 epi | Lingual nerve block | 100 healthy patients who needed procedures under local anesthesia in the mandibular region | Not stated | Unclear |
| India | Long buccal block | ||||||
| Parallel RCT | |||||||
| (n = 100) | |||||||
| (n = 50 in each group) | |||||||
| Malamed et al. [5] 2013 | N/A | 1.8 ml 2% lidocaine with 1:100,000 epi and 8.4% sodium bicarbonate (9:1 ratio) | 1.8 ml 2% lidocaine with 1:100,000 epi | N/A | Healthy volunteers | Gender: N/A | Unclear |
| USA | 41.6 years | ||||||
| Split mouth | [23–76 years] | ||||||
| n = 18 | |||||||
| Phero et al. [13] 2017 | N/A | 4 ml 2% lidocaine with 1:100,000 epi and 8.4% sodium bicarbonate (9:1 ratio) | 4 ml 2% lidocaine with 1:100,000 epi | No co-intervention | Two sessions at the UNC Oral and Maxillofacial Surgery Clinic, ASA I | 11F/12M | Unclear |
| USA, | Exclusion: Allergy to lidocaine class of anesthetic drugs, local anesthetic drug use in past week, current symptoms teeth or oral mucosa | Median = 21 years | |||||
| split mouth | [18–30 years] | ||||||
| (n = 23) | |||||||
| Saatchi et al. [14] 2015 | N/A | 3.6 ml of 2% lidocaine with 1:80,000 epi and 8.4% sodium bicarbonate (9:1 ratio) | 3.6 ml 2% lidocaine with 1:80,000 epi in which 0.36ml lidocaine was replaced by distilled water | N/A | Vital mandibular posterior tooth with active pain and a long response to cold testing with Endo-Frost cold spray | 46F/34M | Unclear |
| Iran, | 35 years in buffered group | ||||||
| Parallel RCT | 36 years in nonbuffered group | ||||||
| (n = 80) | [20–55 years] | ||||||
| (40 in each group) | |||||||
| Schellenberg et al. [15] 2015 | N/A | 2.8ml 4% lidocaine with 1:100,000 epi and 8.4% sodium bicarbonate (9:1 ratio) (n = 50) | 2.8ml 4% lidocaine with 1:100,000 epinephrine (n = 50) | 0.9 ml non-buffered 2% lidocaine with 1:100,000 epi buccal soft tissue anesthesia | Emergency patients | 61F/39M | Unclear |
| USA, | Good health | 35 years in buffered group | |||||
| RCT | Have a vital mandibular posterior tooth with actively experiencing moderate to severe pain Had a prolonged response to cold testing with Endo-Ice | 36 years in non-buffered group | |||||
| (n = 100) | [18–64 years] | ||||||
| (50 in each group) | |||||||
| Tavaka [16] 2013 | N/A | 1.7 ml 2% lidocaine, with 1:100,000 epi and 8.4% sodium bicarbonate (ratio not stated, the onset system) | 1.7 ml 2% lidocaine, with 1:100,000 epi | Long buccal nerve block with separated VAS units for this injection | Informed consent provided 9-12 years of age | 10F/10M | Unclear |
| USA, | Comprehend the visual analog scale, numeric rating scale, and verbal rating scale | [9–12 years] | |||||
| Split mouth | Present moderate mandibular dental disease bilaterally Have 4 to 7 natural teeth present in each mandibular quadrant with moderate dental disease on at least one tooth | ||||||
| (n = 20) | Be willing to attend the clinic for 3 or more appointments | ||||||
| Exclusion: Received an anesthetic, analgesic or sedative within 24 hours prior to the therapy appointments | |||||||
| Warren et al. [17] 2017 | N/A | 4.4 ml of 1% lidocaine with 1:100,000 epi and 8.4% sodium bicarbonate (9:1 ratio) | 4 ml 2% lidocaine with 1:100,000 epi | Lingual and Long buccal nerve block | Treated at the oral and maxillofacial surgery clinic 2 weeks apart, ASA I | 12F/11M | Unclear |
| USA, | Exclusion: Allergy to lidocaine class of anesthetic drugs, local anesthetic drug use in past week, current symptoms teeth or oral mucosa | Median = 25 years | |||||
| Splint mouth | [18–30 years] | ||||||
| (n = 23) | |||||||
| Whitcomb et al. [18] 2010 | N/A | 3.6 ml 2% lidocaine with 1 : 100,000 epi and 8.4% sodium bicarbonate (5:1 ratio) | 3.6 ml 2% lidocaine with 1:100,000 epi | N/A | Good health | 12 F/ 28 M | Unclear |
| USA, | Not taking any medications that would alter their perception of pain | 26 years | |||||
| Split mouth | [18–38 years] | ||||||
| (n = 40) |
Table 2
Summary of outcomes included in meta-analyses
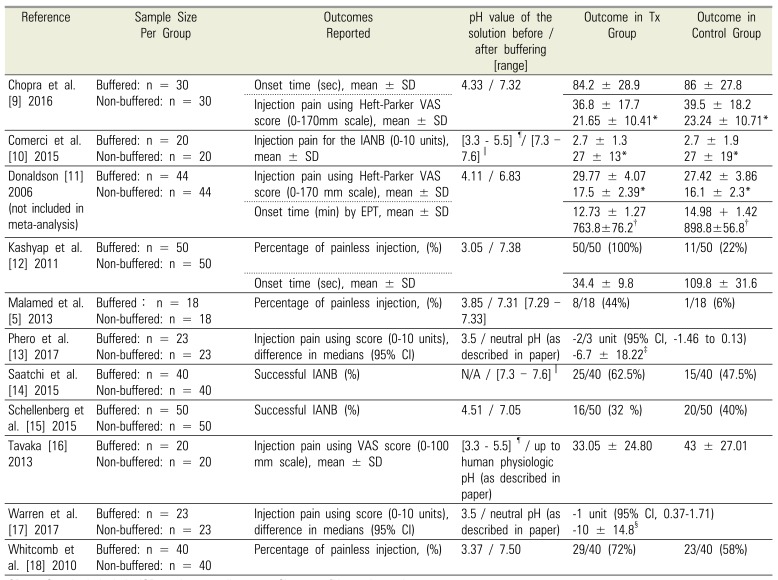
| Reference | Sample Size Per Group | Outcomes Reported | pH value of the solution before / after buffering [range] | Outcome in Tx Group | Outcome in Control Group |
|---|---|---|---|---|---|
| Chopra et al. [9] 2016 | Buffered: n = 30 | Onset time (sec), mean ± SD | 4.33 / 7.32 | 84.2 ± 28.9 | 86 ± 27.8 |
| Non-buffered: n = 30 | Injection pain using Heft-Parker VAS score (0–170mm scale), mean ± SD | 36.8 ± 17.7 | 39.5 ± 18.2 | ||
| 21.65 ± 10.41* | 23.24 ± 10.71* | ||||
| Comerci et al. [10] 2015 | Buffered: n = 20 | Injection pain for the IANB (0–10 units), mean ± SD | [3.3–5.5]¶/ [7.3–7.6]∥ | 2.7 ± 1.3 | 2.7 ± 1.9 |
| Non-buffered: n = 20 | 27 ± 13* | 27 ± 19* | |||
| Donaldson [11] 2006 (not included in meta-analysis) | Buffered: n = 44 | Injection pain using Heft-Parker VAS score (0-170 mm scale), mean ± SD | 4.11 / 6.83 | 29.77 ± 4.07 | 27.42 ± 3.86 |
| Non-buffered: n = 44 | 17.5 ± 2.39* | 16.1 ± 2.3* | |||
| Onset time (min) by EPT, mean ± SD | 12.73 ± 1.27 | 14.98 + 1.42 | |||
| 763.8±76.2† | 898.8±56.8† | ||||
| Kashyap et al. [12] 2011 | Buffered: n = 50 | Percentage of painless injection, (%) | 3.05 / 7.38 | 50/50 (100%) | 11/50 (22%) |
| Non-buffered: n = 50 | Onset time (sec), mean ± SD | 34.4 ± 9.8 | 109.8 ± 31.6 | ||
| Malamed et al. [5] 2013 | Buffered: n = 18 | Percentage of painless injection, (%) | 3.85 / 7.31 [7.29–7.33] | 8/18 (44%) | 1/18 (6%) |
| Non-buffered: n = 18 | |||||
| Phero et al. [13] 2017 | Buffered: n = 23 | Injection pain using score (0–10 units), difference in medians (95% CI) | 3.5 / neutral pH (as described in paper) | −2/3 unit (95% CI, −1.46 to 0.13) | |
| Non-buffered: n = 23 | −6.7 ± 18.22‡ | ||||
| Saatchi et al. [14] 2015 | Buffered: n = 40 | Successful IANB (%) | N/A / [7.3–7.6]∥ | 25/40 (62.5%) | 15/40 (47.5%) |
| Non-buffered: n = 40 | |||||
| Schellenberg et al. [15] 2015 | Buffered: n = 50 | Successful IANB (%) | 4.51 / 7.05 | 16/50 (32%) | 20/50 (40%) |
| Non-buffered: n = 50 | |||||
| Tavaka [16] 2013 | Buffered: n = 20 | Injection pain using VAS score (0–100 mm scale), mean ± SD | [3.3–5.5]¶ / up to human physiologic pH (as described in paper) | 33.05 ± 24.80 | 43 ± 27.01 |
| Non-buffered: n = 20 | |||||
| Warren et al. [17] 2017 | Buffered: n = 23 | Injection pain using score (0–10 units), difference in medians (95% CI) | 3.5 / neutral pH (as described in paper) | −1 unit (95% CI, 0.37–1.71) | |
| Non-buffered: n = 23 | −10 ± 14.8§ | ||||
| Whitcomb et al. [18] 2010 | Buffered: n = 40 | Percentage of painless injection, (%) | 3.37 / 7.50 | 29/40 (72%) | 23/40 (58%) |
| Non-buffered: n = 40 | |||||
SD = Standard deviationIQR = interquartile range CI = confidence interval
*After converting original VAS to 100mm scale
†After converting from minutes to seconds
‡After converting median difference and IQR to mean difference ± SD
§After converting median difference and 95% CI to mean difference ± SD
∥Based on Frank et al. [12]
¶Based on the manufacturer DENTSPLY International
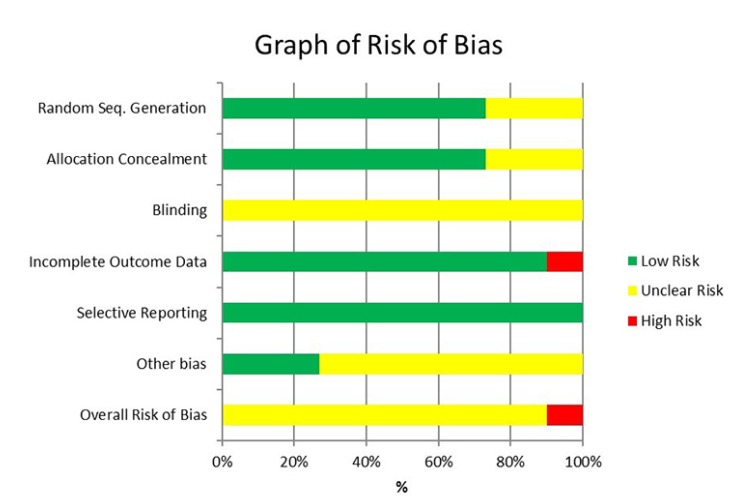
6. Assessment of risk of bias in RCTs
Table 3
Summary of risk of bias for eligible RCT studies
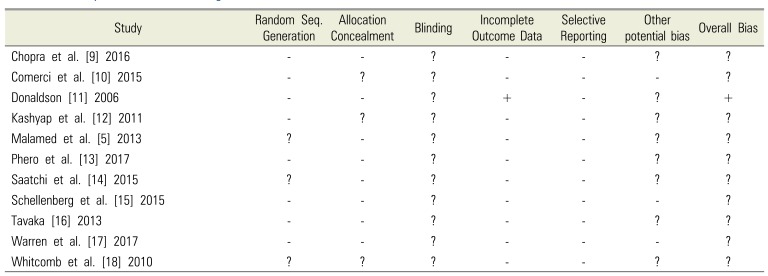
| Study | Random Seq. Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Reporting | Other potential bias | Overall Bias |
|---|---|---|---|---|---|---|---|
| Chopra et al. [9] 2016 | - | - | ? | - | - | ? | ? |
| Comerci et al. [10] 2015 | - | ? | ? | - | - | - | ? |
| Donaldson [11] 2006 | - | - | ? | + | - | ? | + |
| Kashyap et al. [12] 2011 | - | ? | ? | - | - | ? | ? |
| Malamed et al. [5] 2013 | ? | - | ? | - | - | ? | ? |
| Phero et al. [13] 2017 | - | - | ? | - | - | ? | ? |
| Saatchi et al. [14] 2015 | ? | - | ? | - | - | ? | ? |
| Schellenberg et al. [15] 2015 | - | - | ? | - | - | - | ? |
| Tavaka [16] 2013 | - | - | ? | - | - | ? | ? |
| Warren et al. [17] 2017 | - | - | ? | - | - | - | ? |
| Whitcomb et al. [18] 2010 | ? | ? | ? | - | - | ? | ? |
7. Statistical analyses
8. Levels of evidence and summary of the review findings
RESULTS
1. Results of the search
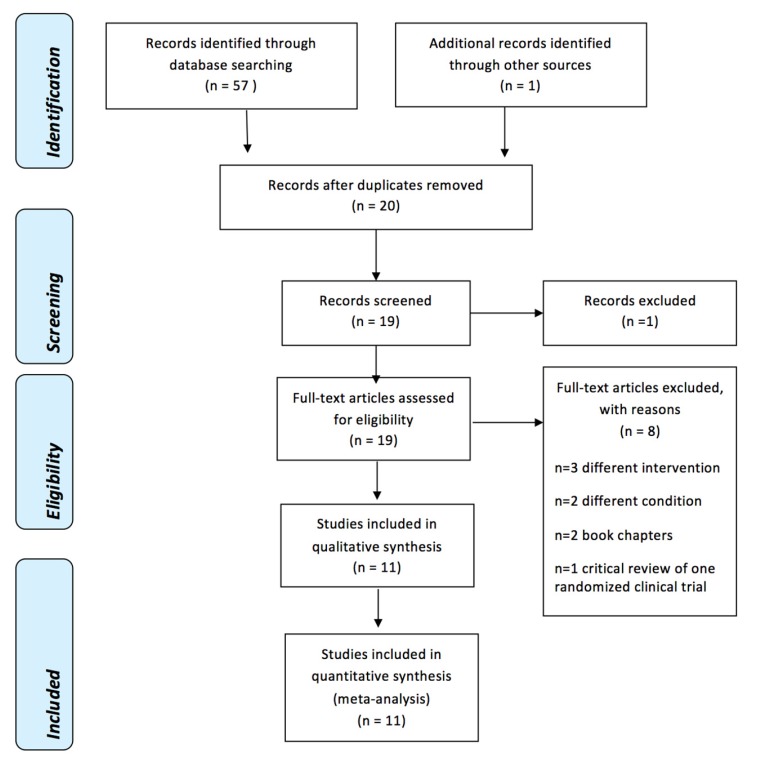 | Fig. 2PRISMA Flow Diagram [33] |
2. Included studies
3. Effects of interventions
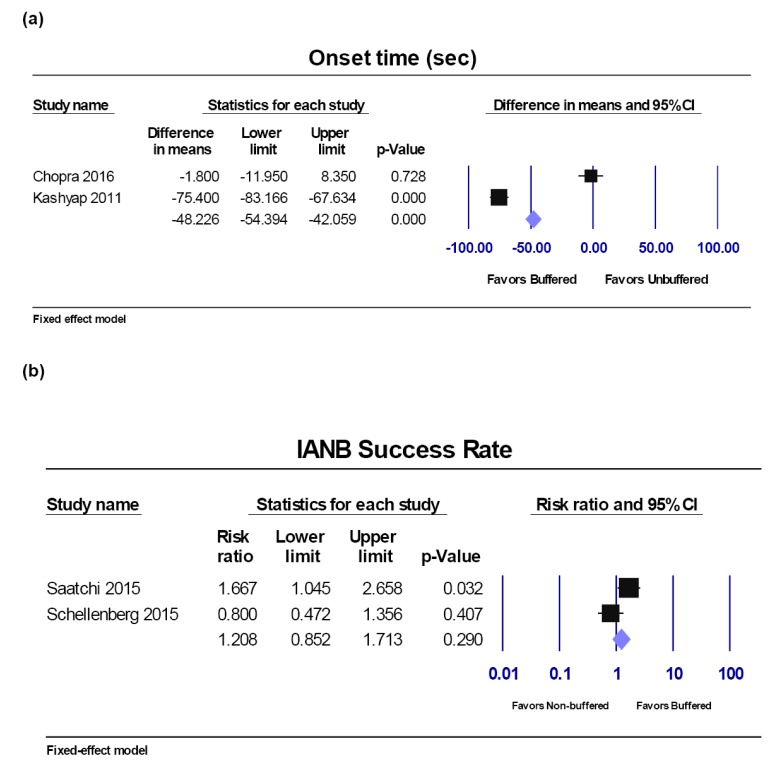
Onset time.
Success rate of IANB.
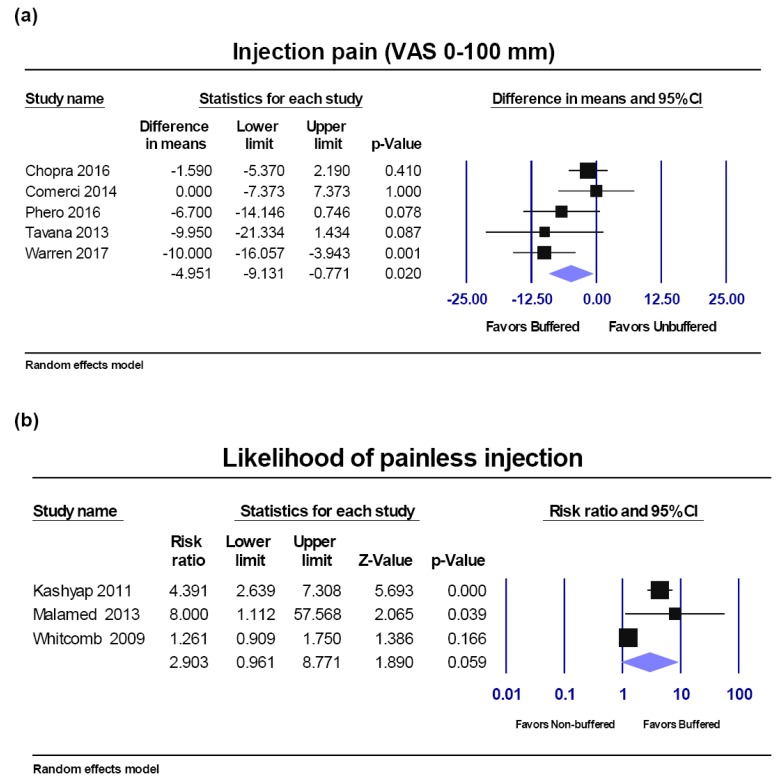
VAS score injection pain.
VAS pain sensitivity analyses:
a) High risk study and low pH:
b) Symptomatic patients:
c) Pediatric patients:
Percentage of painless injection.
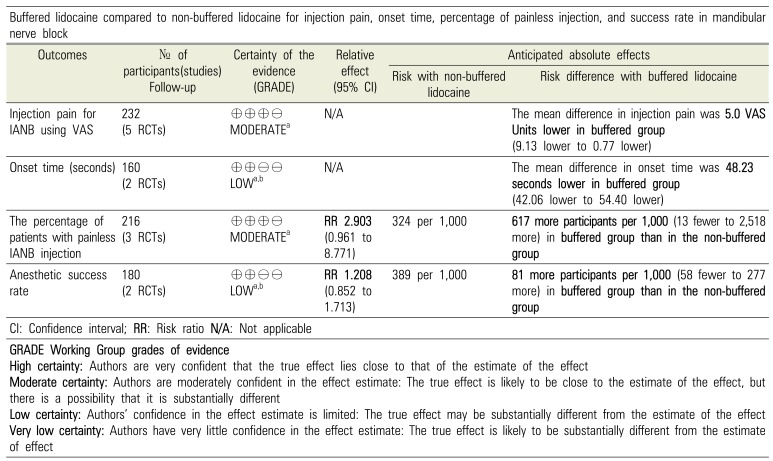
Levels of evidence and summary of the review findings (according to the GRADE recommendation).
Table 4
Summary of the evidence and quality of the findings (GRADE).[34]




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download