Abstract
Background
The number of patients with Alzheimer's disease is growing worldwide, and the proportion of patients requiring dental treatment under general anesthesia increases with increasing severity of the disease. However, outpatient anesthesia management for these patients involves great risks, as most patients with Alzheimer's disease are old and may show reduced cardiopulmonary functions and have cognitive disorders.
Methods
This study retrospectively investigated 43 patients with Alzheimer's disease who received outpatient anesthesia for dental treatment between 2012–2017. Pre-anesthesia patient evaluation, dental treatment details, anesthetics dose, blood pressure, duration and procedure of anesthesia, and post-recovery management were analyzed and compared between patients who underwent general anesthesia or intravenous sedation.
Results
Mean age of patients was about 70 years; mean duration of Alzheimer's disease since diagnosis was 6.3 years. Severity was assessed using the global deterioration scale; 62.8% of patients were in level ≥ 6. Mean duration of anesthesia was 178 minutes for general anesthesia and 85 minutes for intravenous sedation. Mean recovery time was 65 minutes. Eleven patients underwent intravenous sedation using propofol, and 22/32 cases involved total intravenous anesthesia using propofol and remifentanil. Anesthesia was maintained with desflurane for other patients. While maintaining anesthesia, inotropic and atropine were used for eight and four patients, respectively. No patient developed postoperative delirium. All patients were discharged without complications.
Dementia, which is one of major neurocognitive disorders (MNCD), is a non-congenital cognitive disorder characterized by speaking difficulty and reduced memory, problem-solving ability, and ability to carry on with daily living, as well as loss of socio-occupational functions over time [12]. The most common cause of dementia is Alzheimer's disease (AD) (60-80%), and other causes include vascular (10%) and traumatic brain damage as well as systemic diseases, such as Parkinson's disease [2].
The level of disability for dementia patients increases with increasing duration of disease, and patients with severe disease eventually lose the ability to adequately perform oral hygiene on their own, which increases the risk for caries or periodontal diseases [3]. Furthermore, these patients have difficulty in describing their symptoms after developing oral problems, and even if they visit a dentist, the difficulty in communication hinders effective treatment [45]. As patients with MNCD are largely uncooperative, performing appropriate oral examination is challenging, and due to the risks and difficulty of local anesthesia, intravenous sedation (IVS) or general anesthesia (GA) are generally needed [6].
Dental treatment under GA enables dentists to effectively treat patients because they are immobile. Furthermore, safety may be improved by using appropriate doses and treatment based on a pre-procedural assessment of patient's systemic disease status and dental treatment-related risks [7]. In addition, dental treatment usually involves outpatient general anesthesia, which allows patients to receive all necessary dental treatments under GA in one day and return home on the same day, thus avoiding any inconveniences and saving costs and time involved in visiting the clinic multiple times [8].
However, this group of patients is older in age and has multiple comorbidities, which increases the risk of complications of GA, including reduced cardiopulmonary functions [9]. Furthermore, preoperative tests cannot be adequately performed because patients tend to be uncooperative. Anesthetic management for patients with AD is not familiar with the anesthesiologist due to the scant number of reports on outpatient anesthesia [10]. In this article, this study aims to analyze data on dental care under outpatient GA or IVS for AD patients at the Seoul National University Dental Hospital (SNUDH), in an attempt to contribute to effective outpatient anesthesia management for severe AD patients.
This study was approved by the Institutional Review Board (IRB-No S-D20170049). Medical records of AD patients who were treated under outpatient GA or IVS at the Special Care Clinic of the SNUDH between January 1, 2012 and November 30, 2017 were obtained and analyzed.
After analyzing the outpatient anesthesia list, we excluded patients with MNCD caused by cerebrovascular dysfunction, such as cerebral infarction, hemorrhage, patients with brain tumor, and searched for patients who were only diagnosed with AD at neurology. Patients with incomplete medical records were excluded from the analysis (Fig. 1).
In the hospital's electronic medical record database, we also analyzed each patient's dental treatment record, pre-anesthesia assessment record (sex, age, duration of Alzheimer's disease or dementia, current stage, and type of comorbidity), anesthesia and recovery room records (drug used for anesthesia induction, cooperation, airway management, total duration of anesthesia, duration of stay in the recovery room, and side effects and complications observed in the recovery room), next-day follow-up record data by phone (presence of complications after returning home), dental treatment details and duration of dental care. In addition, we investigated any differences in relation to the type of anesthetic used as well as between GA and IVS.
Vital sign data(blood pressure (BP), ECG, oxygen saturation, and capnography) during anesthesia that were monitored at five-minute intervals (Sola 8000M, GE, USA) and relayed to the hospital's server were used for analysis. Bispectral index (BIS, USA) and target-controlled infusion (TCI) data were obtained from electronic medical records, which were manually written based on the number shown on the infusion pump (Fresenius Orchestra, Fresenius Kabi, Germany).
For data analysis, each category was presented as the number of patients or procedures, and where necessary, as mean and standard deviation or number with percentage. With regard to medical history records, missing records or incomplete records were excluded, and only well-recorded data were included in the analysis.
Of 1,132 adult patients who received dental treatment under GA or IVS at the Special Care Clinic of the SNUDH during a period of six years from January 2012 to November 2017, 43 (3.8%) were AD patients. GA for dental treatment was performed in 32 of these AD patients, while IVS was performed 11 patients. One patient received four rounds of dental treatment, and six patients received two rounds of treatment; all the remaining patients received only one dental treatment. The mean age of the patients was about 70 years, and the mean duration of AD was 6.3 years. It took about 70 days from the first examination at our hospital to dental treatment under outpatient anesthesia (Table 1).
The severity of disease in the patients who underwent GA or IVS was assessed using the global deterioration scale (GDS) [11] based on the patients' attitudes and caregivers' statements on the day of the first examination. Twenty-seven patients had a moderately severe dementia (level 6) or higher, accounting for 62.8%. Ten out of a total of 43 patients were taking anti-hypertensive drugs, and there were three patients with diabetes, eight patients with Parkinson's disease, and two patients with a respiratory disorder. None of the patients had a severe disease that would contraindicate GA. A total of 38 out of 43 patients were classified as American Society of Anesthesiologists (ASA) physical class 2 (81.8%), and five patients were classified as ASA class 3 (18.2%).
Thirty patients underwent blood laboratory tests, electrocardiogram (ECG), and chest X-ray prior to surgery. However, three patients, who displayed needle phobia who had no notable findings in past health examinations or neurological tests, underwent blood tests on the same day after induction of anesthesia.
The method of anesthesia was usually determined based on the patient's state and treatment plan. The Alzheimer GDS level and the proportion of comorbidities were greater in patients who we determined should undergo treatment under IVS than those undergoing GA (Table 2).
Cooperation during induction was assessed by the anesthesiologist on the day of the treatment. In most cases, the anesthesiologist was able to start an intravenous (IV) catheterization without coercive physical restraints (cooperative grade 1, and 2, 81.3%). However, 14 patients who were assessed to be cooperative had severe dementia (Alzheimer GDS level 7) and were incapable of resisting or responding to the attempted treatment (Table 3) [12]. For five patients for whom IV catheter insertion was difficult due to poor cooperation, anesthesia was induced via inhalation of sevoflurane. For all the patients who underwent IVS, sedation was induced and maintained via target-controlled infusion (TCI) with propofol. For 18 of the patients who underwent GA, anesthesia was induced via TCI with propofol and remifentanil, and maintained via total intravenous anesthesia (TIVA). For four patients who received sevoflurane for induction of anesthesia, anesthesia was maintained with TIVA, and for ten patients, including nine patients for whom thiopental was used for induction, anesthesia was maintained with desflurane. When propofol was used, the induction dose was significantly different between those who underwent GA and those who underwent IVS, where the induction dose was significantly higher for patients who underwent GA (P < 0.001) (Table 4).
With GA, the airway was maintained through nasotracheal intubation. One patient had a difficult airway, for whom a fiberscopic intubation was performed. For a patient with IVS, O2 was administered via a nasal cannula at 2 L/min. The degree of sedation was confirmed with BIS, and propofol concentration was adjusted accordingly. Treatment was performed in a deep sedative state where the patient is unconscious. Among patients who underwent GA, inotropic agents (e.g., ephedrine) were administered due to a severe drop of BP in eight patients, and four patients needed anti-arrhythmics due to severe bradycardia or tachycardia. BP and pulse rate were relatively stable in patients who underwent IVS. BIS was maintained below 60 for patients undergoing GA and between 60-80 for patients undergoing IVS (Fig. 2).
The duration of anesthesia with GA was twice that of IVS. After the completion of surgery, administration of the anesthetics was stopped, and recovery of consciousness and spontaneous breathing were confirmed before extubation, after which the patients were transferred to the recovery room. This took about an average of 18 min, whereas it took about 13 min for patients who underwent IVS to recover consciousness. In the recovery room, the patients were confirmed to have recovered to their pre-anesthesia states and to have stable vital signs before discharge. The duration of stay in the recovery room differed by about 20 min between the GA and IVS groups (Table 5). During recovery, postoperative delirium (POD) was not observed, and there were no complications requiring hospitalization. Fifty percent of the patients returned to a long-term care hospital, while the remaining 50% of the patients returned to their homes. When returning home, 75% of the patients used their own cars, 15.7% used an ambulance, and 9.3% used a taxi. At the time of returning home, 11.6% were in a drowsy state; however, the remaining patients had recovered their pre-anesthesia states. About 5% of the patients stated that they slept longer than usual on the first day after returning home, and there were no cases of nausea or vomiting. During an over-the-phone check-up the next day, five patients complained a mild fever and three had persistent oozing at the extraction site; however, none of the patients developed complications requiring a hospital visit.
The most commonly performed dental treatment for AD patients was tooth extraction (92.7%) and caries treatment (43.9%). The mean number of extracted teeth among the patients who received tooth extraction was 5.9 ± 4.9. The mean number of dental caries among the patients who underwent caries treatment was 3.4 ± 2.1.
The worldwide prevalence of dementia is about 5–7% of the elderly population aged 60 years or older, and at the current growth rate, the prevalence is estimated to double every 20 years [13]. In South Korea, about 9.2% of the elderly population aged 65 years or older have dementia, and the prevalence rises with advancing age [14].
Although the exact cause of Alzheimer's AD has not yet been elucidated, a mutation of the amyloid precursor protein gene located in chromosome 21 is known as the cause of early-onset familial Alzheimer's disease, in which dementia develops before the age of 65 years. A mutation of the presenilin 1 gene in chromosome 14 as well as a mutation of the presenilin 2 gene in chromosome 1 are also known as some causes of AD [15]. With regard to dementia that sporadically appears after the age of 65, the apolipoprotein E4 allele in chromosome 19 is known to be a risk factor. AD is a syndrome caused by an interaction of progressive neurological degeneration, genetic risk factors, and environmental risk factors, as opposed to a disease caused by a single specific cause. AD patients demonstrate behavioral changes, including disordered cognitive functions and irreversible memory disturbance, delusion, reduced social adjustment, verbal disability, walking difficulty, and reduced motor coordination similar to that of Parkinson's disease [16].
The treatment trends at our hospital show that the annual number of cases receiving treatment is rising; however, the treatment is generally focused on tooth extraction and caries treatment. As AD progresses, oral health is exacerbated, particularly, the prevalence of periodontal diseases is reported to be higher among AD patients compared to the healthy population, and dental caries is known to proportionately rise with the degree of exacerbation of dementia symptoms [17]. Furthermore, drugs that are taken to mitigate neurological symptoms not only increase dental caries but also may cause mucositis, gingival hypertrophy, and intraoral ulcer [18]. Such poor oral health and increase in oral diseases induce pain and discomfort, and changes of swallowing habits may have a toll on self-esteem. Therefore, appropriate dental intervention is essential for AD patients to lower pain and oral diseases and maintain adequate oral health. This would in turn improve quality of life and hinder worsening of oral states in the later stages of dementia [19].
However, dental treatment for moderately to severe AD patients must be performed under IVS or GA because these patients have no insight into their dental diseases and demonstrate poor cooperation [6]. Treatment under IVS or GA is performed while the patient's movements are reduced or eliminated, so quality dental care can be provided irrespective of the patient's cooperation. Furthermore, the amount of treatment that can be performed in one session as well as the time of one session of treatment can be increased, resulting in fewer hospital visits. In addition, patients do not have to undergo a painful or bad experience, as the treatment is performed while the patient is unconscious, which helps patients maintain a positive attitude toward future dental treatments [8].
Anesthesia for AD patients must be performed in adherence to the geriatric standards and with extra precaution, as most AD patients are older adults. The most well-known and confirmed risk involved with GA in relation to AD is age and not AD per se [20]. Patient safety can be enhanced by using appropriate doses and providing appropriate treatment based on a preanesthesia risk assessment by the anesthesiologist. Ten out of the 43 AD patients referred to our hospital (23%) had hypertension, which was in line with the hypertension prevalence in South Korea (22.9%) [21].
The anesthesia method was determined based on the patient's general condition and comorbidity, amount of time needed for dental procedure, and type of techniques used. The durations of anesthesia and procedure were significantly shorter for IVS in our hospital, and this may be attributable to the following factors. First, even though there is no underlying disease, a reduction of functional capacity is a predictor of postoperative pulmonary complications [22], and patients with Alzheimer GDS level 7 fall under this category. Second, aspiration pneumonia is one of the most common causes of mortality among patients with late stage AD, primarily because of reduced consciousness, dysphagia, loss of the gag reflex, and periodontal disease [23]. Prolonging IVS for dental treatment for patients with reduced Gag reflex has no benefits for the patients. When one IVS session was insufficient to complete treatment, we had the patients visit the hospital several times to complete the treatment. GA was performed for patients who could not visit the hospital several times, who were scheduled to undergo a large number of dental extractions, who were in needed of adequate muscle blockade to be treated in the molar region, or who required an analgesic other than propofol due to a temporomandibular joint disease.
Overall, vital signs were maintained at a stable level. but vital sign changes were observed in the 1 hour interval after induction. This was not related to the presence or absence of underlying disease in the patients and occurred in general anesthesia using TIVA (6 out of 8 cases). In GA, propofol requires a higher induction dose than IVS, which can cause a drop in blood pressure and tachycardia. Autonomic dysfunction is accompanied in patients with AD or dementia [24], implying the possibility of excessive hemodynamic change due to drug response. With regard to the correlation between the chosen drug and SBP in TIVA, there is a possibility that the drop in SBP may be inversely proportional to the concentration of remifentanil. Remifentanil is quickly degraded by esterase in blood and tissues; however, the level of esterase declines with aging, with a decrease of about 30% at the age of 80. The volume of distribution also decreases by about 20%, which leads to a higher peak concentration compared to that in younger adults. Further, extra precaution is demanded when bolus-injecting remifentanil, as it may induce severe hypotension and bradycardia [25]. During TIVA, a high-dose drug is injected at the early stage to achieve the initial effect-site concentration, after which the dose is reduced, and this may cause side effects. In fact, when remifentanil is used, reduced blood pressure is maintained for a while even after adjusting the dose after the stimulus is lost. This may explain why inotropics were usually used during TIVA. Atropine, a medication for bradycardia, was also used only in TIVA cases, and patients in these cases are speculated to have developed opioid-induced bradycardia caused by remifentanil.
Inhalation or intravenous agents used for GA are predicted to be associated with AD at the tissue level in animal models [26]. However, there were no differences in maze-solving abilities or motor capacities from those of animal models without exposure [27].
Minimum alveolar concentration (MAC) is defined as “the minimum alveolar concentration of anesthetic at 1 atmosphere that produces immobility in 50 per cent of those patients or animals exposed to a noxious stimulus,” and surgical incision is generally the stimulus in humans. Human studies have reported that aging is associated with a reduction in the MAC. Patients with reduced consciousness due to a brain injury require a lower amount of inhalation anesthetics [28]. However, in animal studies, the presence of pathological changes, that is, neuropathology such as AD, was predicted to induce resistance to hypnotic action caused by inhalation anesthetics [26].
The MAC in the 40-yr-old of sevoflurane and desflurane is 2 and 6 vol%, respectively. According to the formula suggested by Nickealls et al., the The MAC in the 70-yr-old (average age of our patients) of desflurane is 4.98 vol% [29]. The average amount of desflurane used in our hospital was 3.2 vol% ± 0.7. This is a markedly lower concentration than those reported in previous studies, and additional studies are needed to substantiate whether AD patients demonstrate resistance to inhalation anesthetics.
According to the study by Schultz, electroencephalogram (EEG) during total intravenous anesthesia (TIVA) seems to be affected by age. The amount of propofol required while anesthesia is stably maintained is also smaller than that in younger patients, and recovery was also slower [30]. This is correlated with our finding that while the maintenance dose for propofol and desflurane was lower than the generally known maintenance dose, BIS was maintained at 40–60, which is the level for maintaining GA.
When using GA for treatment purposes in AD patients, the benefits of GA must be prioritized over the risks that have not been established. However, it is necessary to avoid low body temperature and isoflurane, which were confirmed by many studies to have an impact [31].
None of our patients showed postoperative delirium (POD) during recovery. Delirium was defined as an acute change in cognition characterized by inattention, fluctuating levels of consciousness, and/or disorganized thinking. In non-cardiac surgeries, the predictors of POD include cognitive disorder, age of 70 years or older, reduced physical capacity, alcoholism, and abnormal blood test results such as electrolyte abnormality [32]. These risk factors are in common with AD patients' conditions, so AD patients are predicted to have a high risk for POD. Intravenous or inhalation anesthetics must be used carefully because mental confusion may be exacerbated in AD patients after administering sedatives or anesthetics [33]. However, this was not the case at our hospital, presumably because of our aggressive nondrug prevention strategies [32]. The primary caregiver was told to stay with the patient from the first recovery room, and a warm environment with appropriate lighting was provided. Hearing aids were provided when necessary. For patients who had their teeth extracted, non-opioid analgesics were provided to control pain if not particularly prohibited. We believe that these factors contributed to the prevention of POD at our institution.
One limitation of this study is that although we collected data from dementia patients for five years, our sample size was below 50. However, we expect to gain more experience in several years based on the current rate of new dementia patients. Furthermore, as with other retrospective studies, our analyses may not be strict due to missing values caused by incomplete medical records data.
Outpatient anesthesia care for AD patients has rarely been studied in the literature. Moreover, this was a great challenge for anesthesiologists, as AD patients are known to have a high risk for POD. There may also be a vague fear of encountering multiple underlying diseases due to the old age of patients. However, based on our experience of performing outpatient anesthesia, patients with AD-type dementia did not particularly have a greater number of comorbidities compared to the general population and also did not develop POD.
In conclusion, this study finds that using smaller drug doses and providing adequate anesthetic management would prevent hazards to AD patients undergoing IVS or GA. Although oral health examination is difficult for AD patients, we believe that their oral health has improved with anesthesia consultation.
References
1. Hugo J, Ganguli M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin Geriatr Med. 2014; 30:421–442. PMID: 25037289.
2. Alzheimer's Association. 2017 Alzheimer's disease facts and figures. Alzheimers Dement. 2017; 13:325–373.
3. Ellefsen B, Holm-Pedersen P, Morse DE, Schroll M, Andersen BB, Waldemar G. Assessing caries increments in elderly patients with and without dementia: A one-year follow-up study. J Am Dent Assoc. 2009; 140:1392–1400. PMID: 19884398.
4. Chen X, Clark JJ, Naorungroj S. Oral health in nursing home residents with different cognitive statuses. Gerodontology. 2013; 30:49–60. PMID: 22364512.

5. Kieser J, Jones G, Borlase G, MacFadyen E. Dental treatment of patients with neurodegenerative disease. N Z Dent J. 1999; 95:130–134. PMID: 10687380.
6. Kocaelli H, Yaltirik M, Yargic LI, Özbas H. Alzheimer's disease and dental management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 93:521–524. PMID: 12075198.

7. Lim MA, Borromeo GL. The use of general anesthesia to facilitate dental treatment in adult patients with special needs. J Dent Anesth Pain Med. 2017; 17:91–103. PMID: 28879336.

8. Caputo AC. Providing deep sedation and general anesthesia for patients with special needs in the dental office-based setting. Spec Care Dentist. 2009; 29:26–30. PMID: 19152565.

9. Rosenthal RA, Kavic SM. Assessment and management of the geriatric patient. Crit Care Med. 2004; 32(4 Suppl):S92–S105. PMID: 15064668.

10. Kim MS, Seo KS, Kim HJ, Han HJ, Shin TJ, Chang J. Dental treatment of a patient with alzheimer disease under ambulatory general anesthesia. J Korean Dent Soc Anesthesiol. 2011; 11:146–152.

11. Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. American journal of psychiatry. Am J Psychiatry. 1982; 139:1136–1139. PMID: 7114305.
12. Seo KS, Shin TJ, Kim HJ, Han HJ, Han JH, Kim HJ, et al. Clinico-statistical analysis of cooperation and anesthetic induction method of dental patients with special needs. J Korean Dent Soc Anesthesiol. 2009; 9:9–16.

13. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013; 9:63–75.e2. PMID: 23305823.

14. Kim YJ, Han JW, So YS, Seo JY, Kim KY, Kim KW. Prevalence and trends of dementia in korea: A systematic review and meta-analysis. J Korean Med Sci. 2014; 29:903–912. PMID: 25045221.

15. Rocchi A, Pellegrini S, Siciliano G, Murri L. Causative and susceptibility genes for alzheimer's disease: A review. Brain Res Bull. 2003; 61:1–24. PMID: 12788204.

16. Selkoe DJ. Alzheimer's disease: Genes, proteins, and therapy. Physiol Rev. 2001; 81:741–766. PMID: 11274343.

17. Warren JJ, Chalmers JM, Levy SM, Blanco VL, Ettinger RL. Oral health of persons with and without dementia attending a geriatric clinic. Spec Care Dentist. 1997; 17:47–53. PMID: 9582699.

18. Turner LN, Balasubramaniam R, Hersh EV, Stoopler ET. Drug therapy in alzheimer disease: An update for the oral health care provider. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106:467–476. PMID: 18928896.

19. Mancini M, Grappasonni I, Scuri S, Amenta F. Oral health in alzheimer's disease: A review. Curr Alzheimer Res. 2010; 7:368–373. PMID: 20043813.

20. Zuo C, Zuo Z. Spine surgery under general anesthesia may not increase the risk of alzheimer's disease. Dement Geriatr Cogn Disord. 2010; 29:233–239. PMID: 20375503.

21. Choi KM, Park HS, Han JH, Lee JS, Lee J, Ryu OH, et al. Prevalence of prehypertension and hypertension in a korean population: Korean national health and nutrition survey 2001. J Hypertens. 2006; 24:1515–1521. PMID: 16877953.

22. Qaseem A, Snow V, Fitterman N, Hornbake ER, Lawrence VA, Smetana GW, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: A guideline from the american college of physicians. Ann Intern Med. 2006; 144:575–580. PMID: 16618955.

23. Kalia M. Dysphagia and aspiration pneumonia in patients with alzheimer's disease. Metabolism. 2003; 52(10 Suppl 2):36–38. PMID: 14577062.

24. Allan LM, Ballard CG, Allen J, Murray A, Davidson AW, McKeith IG, et al. Autonomic dysfunction in dementia. J Neurol Neurosurg Psychiatry. 2007; 78:671–677. PMID: 17178816.

25. Minto CF, Schnider TW, Shafer SL. Pharmacokinetics and pharmacodynamics of remifentanil. II. Model application. Anesthesiology. 1997; 86:24–33. PMID: 9009936.
26. Bianchi SL, Caltagarone BM, Laferla FM, Eckenhoff RG, Kelz MB. Inhaled anesthetic potency in aged alzheimer mice. Anesth Analg. 2010; 110:427–430. PMID: 19820240.

27. Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004; 100:309–314. PMID: 14739805.

28. Aranake A, Mashour GA, Avidan MS. Minimum alveolar concentration: Ongoing relevance and clinical utility. Anaesthesia. 2013; 68:512–522. PMID: 23414556.

29. Nickalls RW, Mapleson WW. Age-related iso-mac charts for isoflurane, sevoflurane and desflurane in man. Br J Anaesth. 2003; 91:170–174. PMID: 12878613.
30. Schultz A, Grouven U, Zander I, Beger FA, Siedenberg M, Schultz B. Age-related effects in the EEG during propofol anaesthesia. Acta Anaesthesiol Scand. 2004; 48:27–34. PMID: 14674970.

31. Papon MA, Whittington RA, El-Khoury NB, Planel E. Alzheimer's disease and anesthesia. Front Neurosci. 2011; 4:272. PMID: 21344011.

32. Rudolph JL, Marcantonio ER. Review articles: Postoperative delirium: Acute change with long-term implications. Anesth Analg. 2011; 112:1202–1211. PMID: 21474660.
33. Fernandez CR, Fields A, Richards T, Kaye AD. Anesthetic considerations in patients with alzheimer's disease. J Clin Anesth. 2003; 15:52–58. PMID: 12657410.

Fig. 1
A flow chart of the study population is presented.
GA = general anesthesia; IV = intravenous sedation.
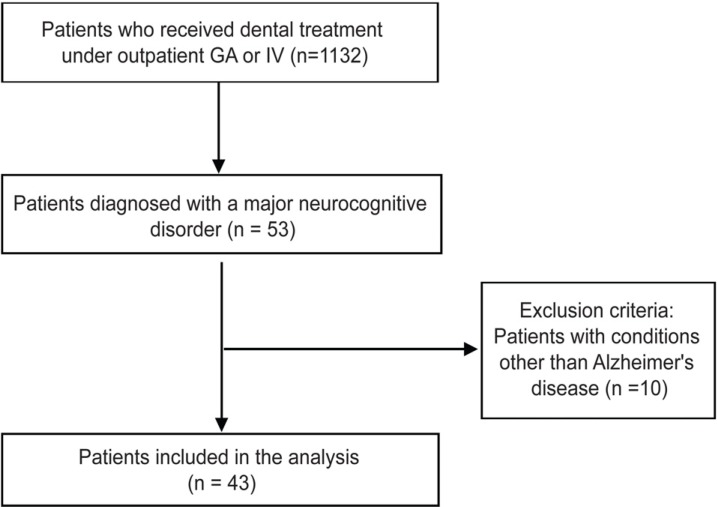
Fig. 2
Trends of monitoring values are shown. Patients were divided into three groups according to the anesthetics used, and trends were observed for each group. Only the one-hour period after induction is presented to all patients. (A) Changes in systolic blood pressure during a one-hour period are shown. The total intravenous anesthesia (TIVA) GA group showed significant changes in systolic blood pressure (SBP) until 30 minutes after the induction (ANOVA test). There was no significant difference in SBP between the GA group and intravenous sedation (IVS) group, (B) Changes in anesthetic dosage during a one-hour period are shown. When viewed in terms of propofol and remifentanil, the two anesthetics used for TIVA GA, remifentanil dose was reduced in response to a drop in SBP about 10 minutes after induction, after which the SBP was restored, (C) Changes in BIS during a one-hour period are shown. The IVS group maintained a high BIS compared to that of the GA group (ANOVA test). GA = general anesthesia, BP = blood pressure. *P < 0.05, ANOVA.
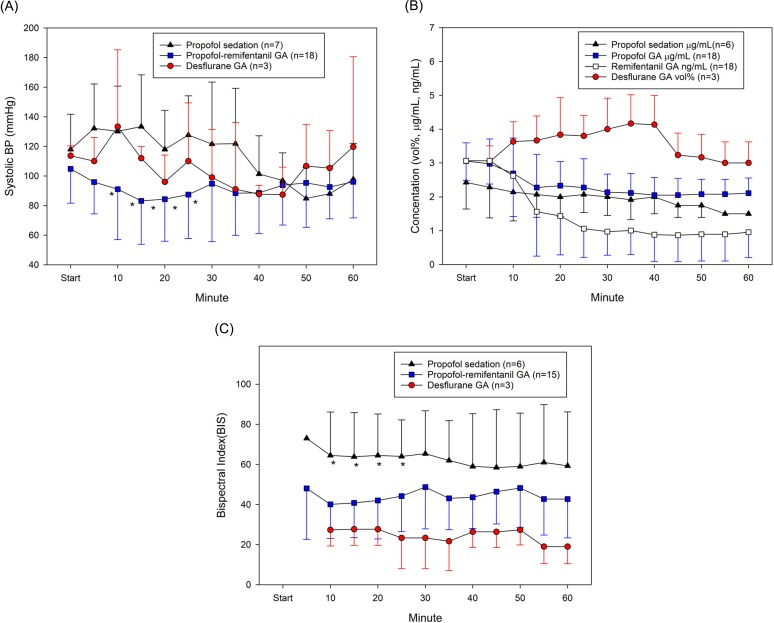
Table 1
Patient characteristics of the study subjects
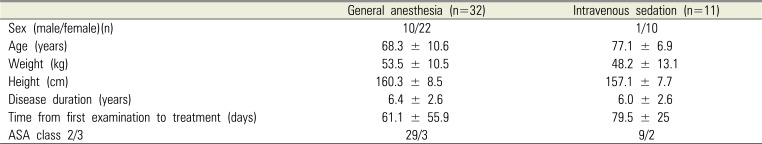
Table 2
Patients' Alzheimer GDS level and choice of anesthesia method
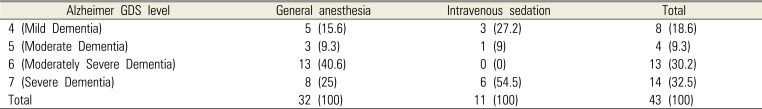
Table 3
Degree of cooperation during induction

Table 4
Anesthesia induction and maintenance dose
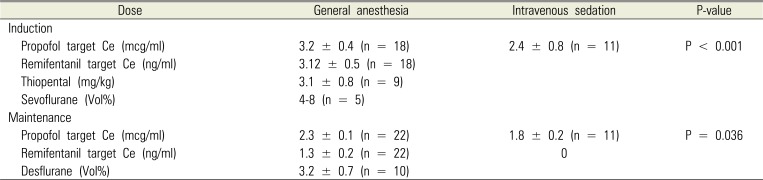
Table 5
Anesthesia and recovery duration





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download